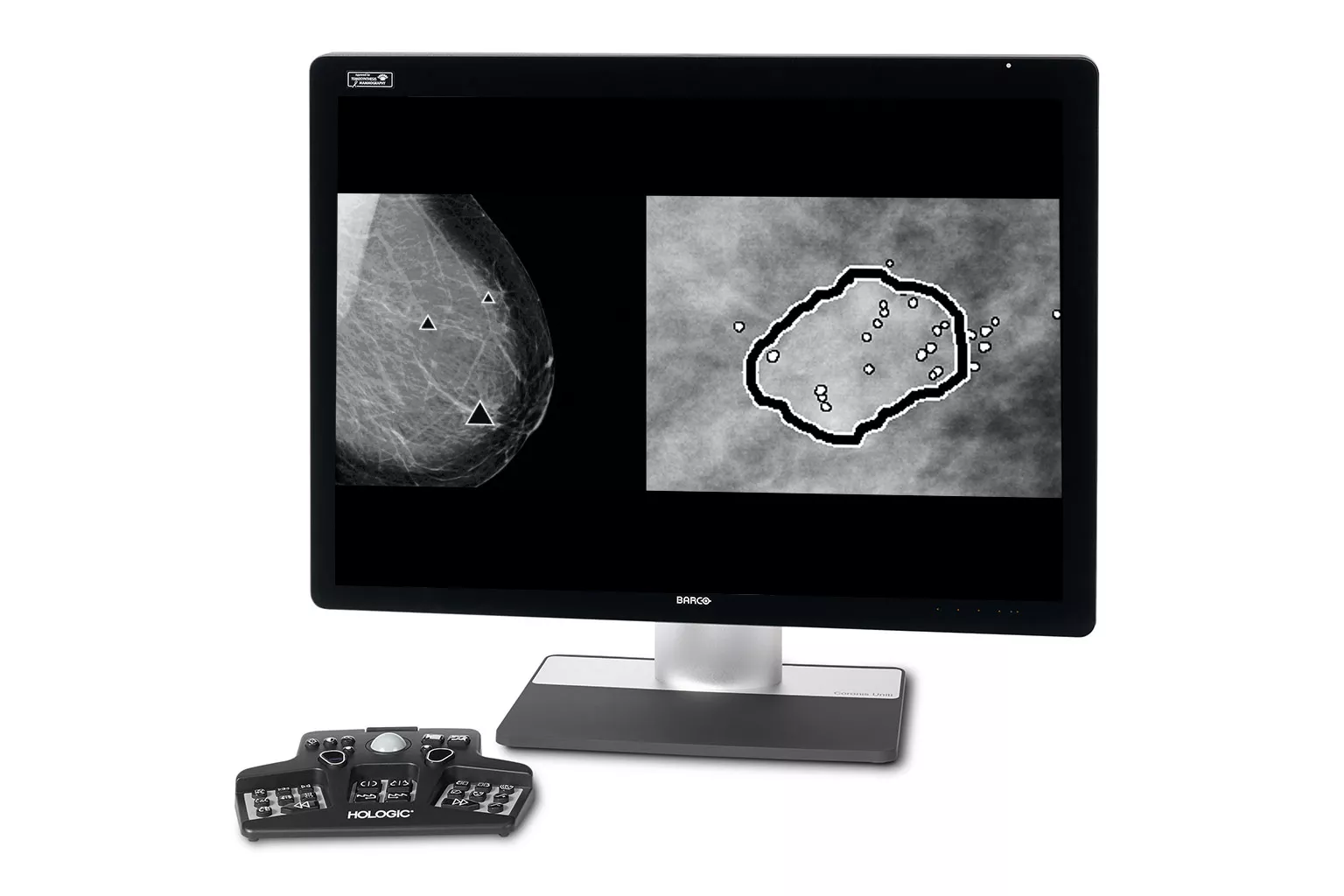
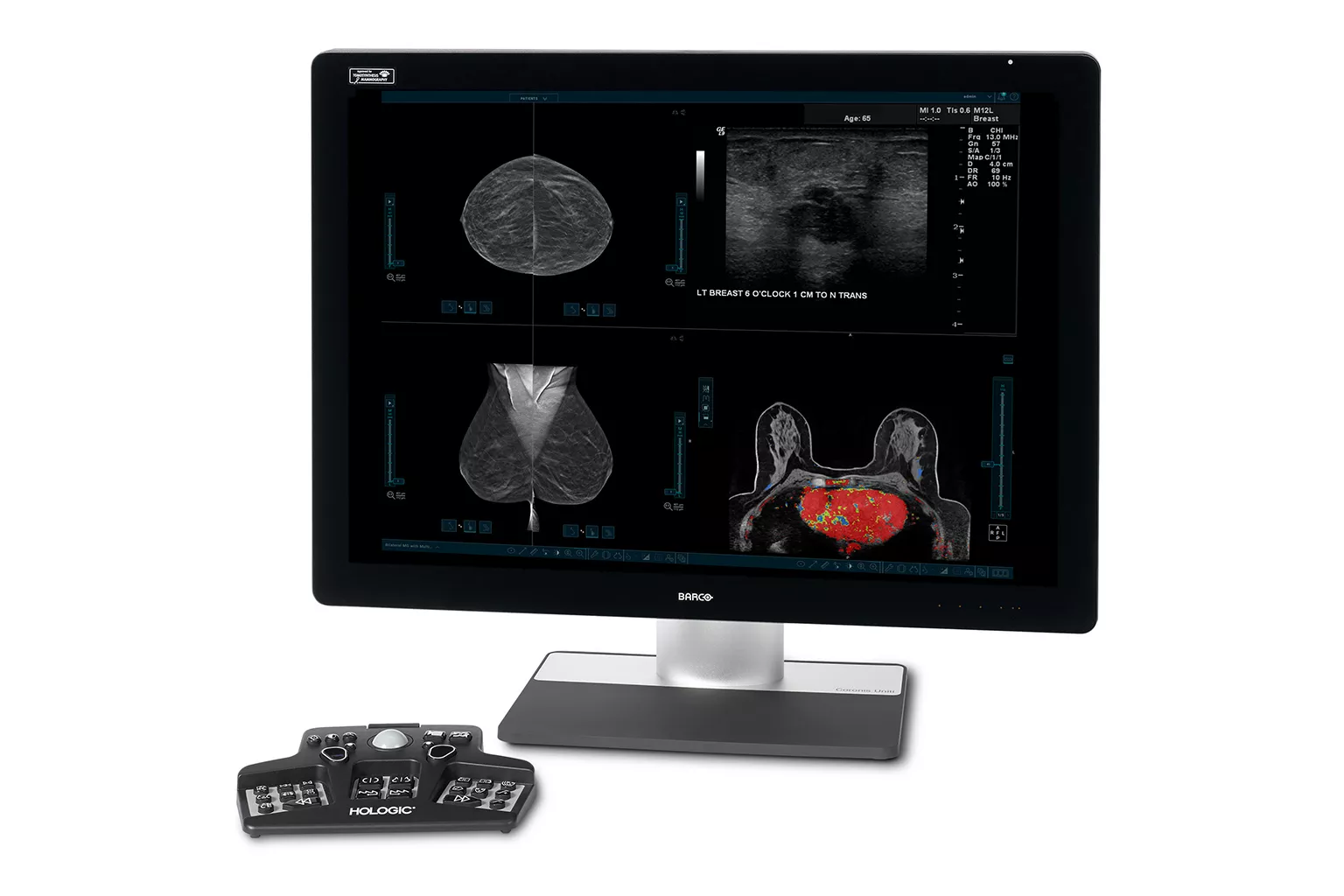
Breast Imaging
Leading the way in breast imaging. Hologic's breast imaging technology includes state-of-the-art mammography systems, and advanced AI and software solutions for sophisticated breast image analysis.
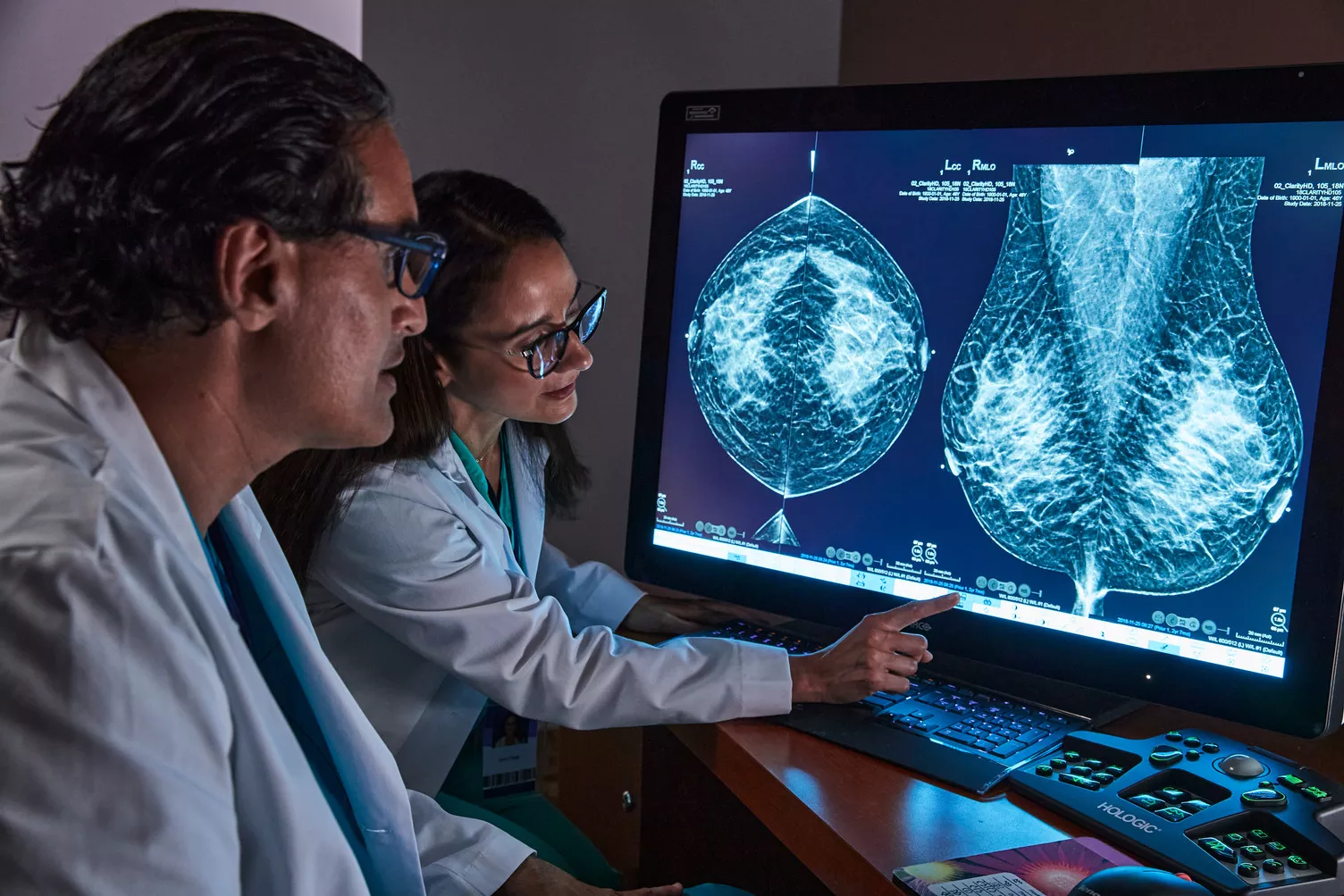
Advance Early Detection & Diagnostic Accuracy
Our integrated breast imaging technologies are designed for superior breast cancer screening1, from mammography systems that prioritize patient comfort and provide workflow enhancements, to leading-edge AI software and modern workstations for proactive and decisive reading.
Cutting-Edge Imaging
Confident Cancer Detection
Compassionate Interventional Solutions
Diagnostic & Data Efficiency
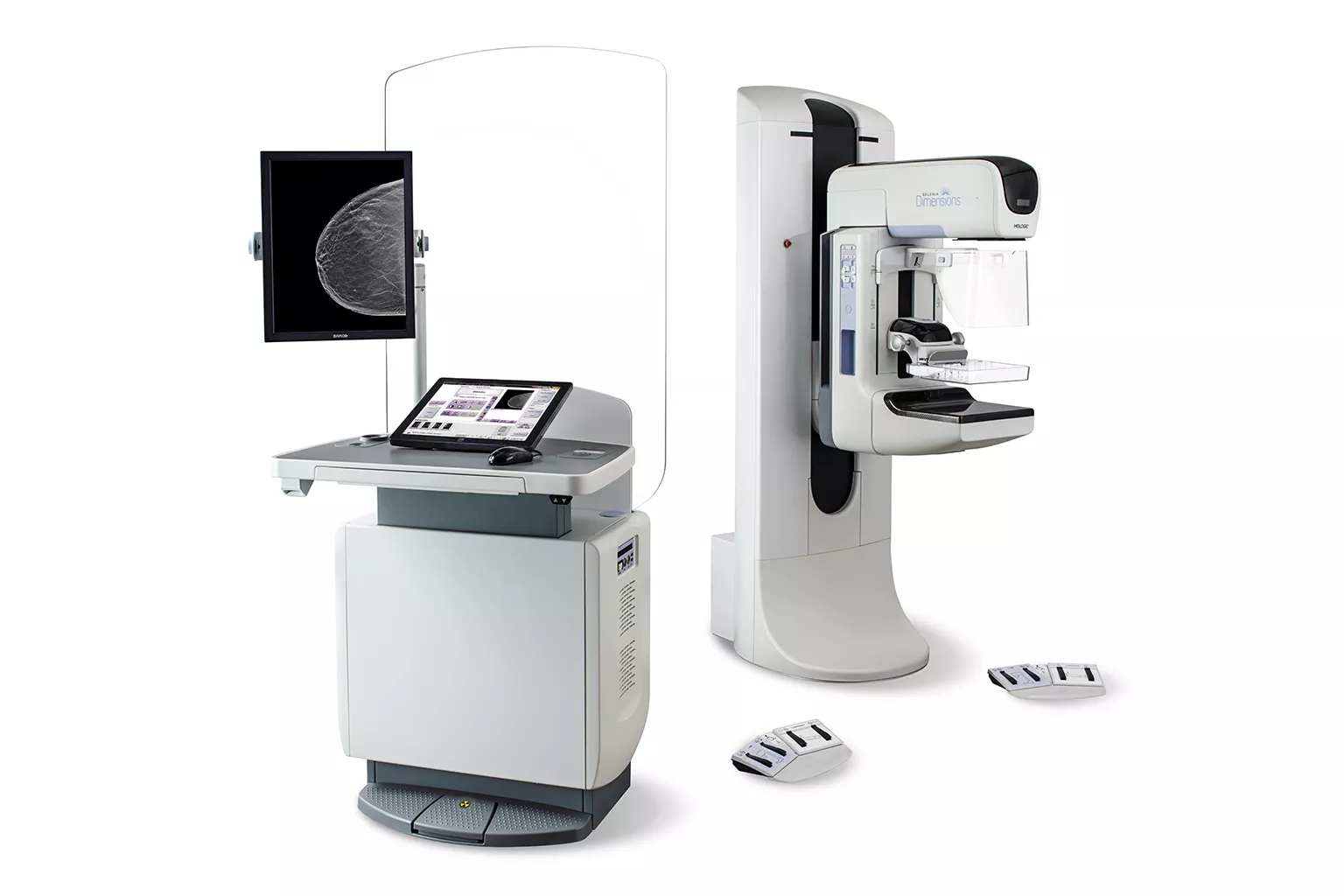
Mammography
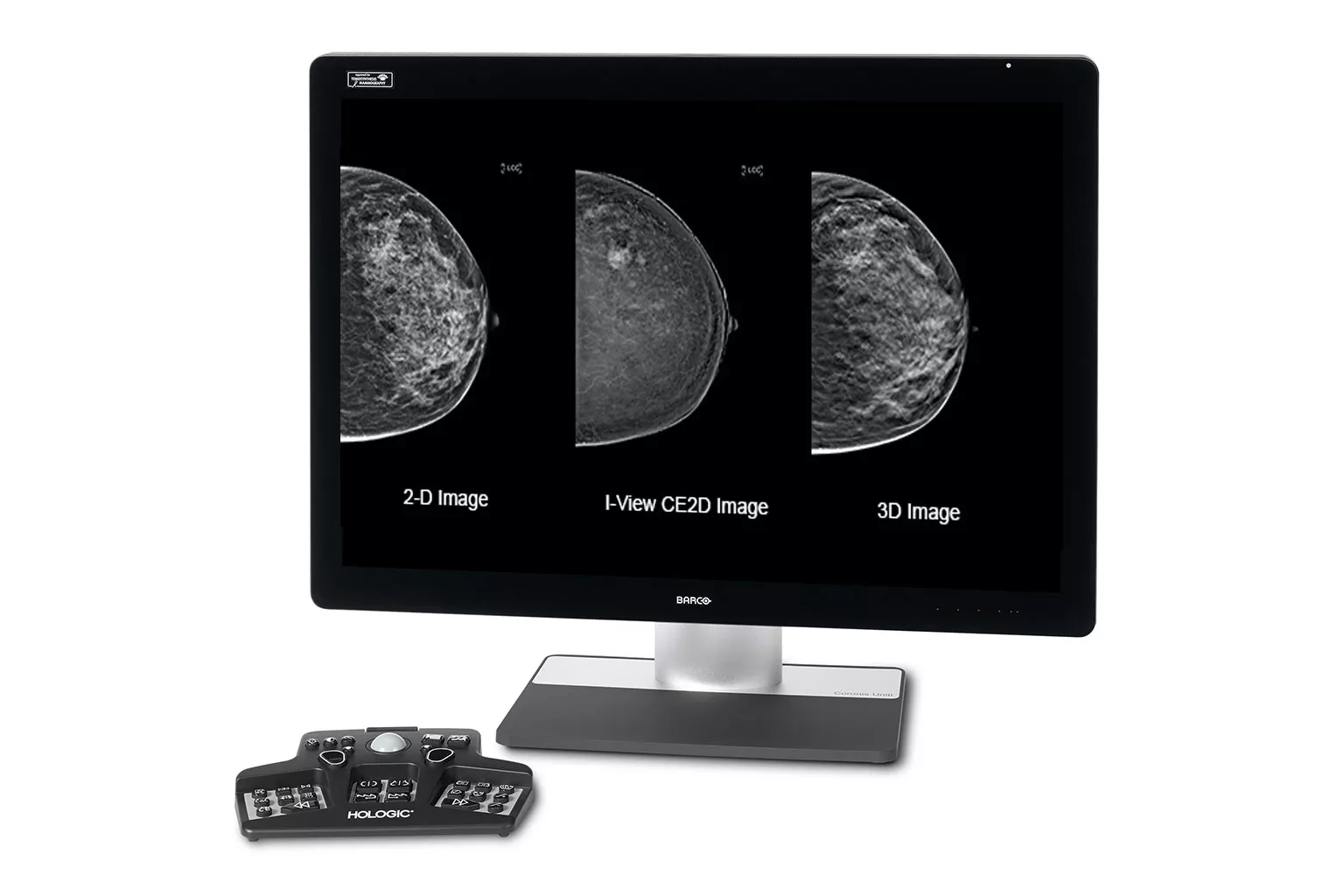
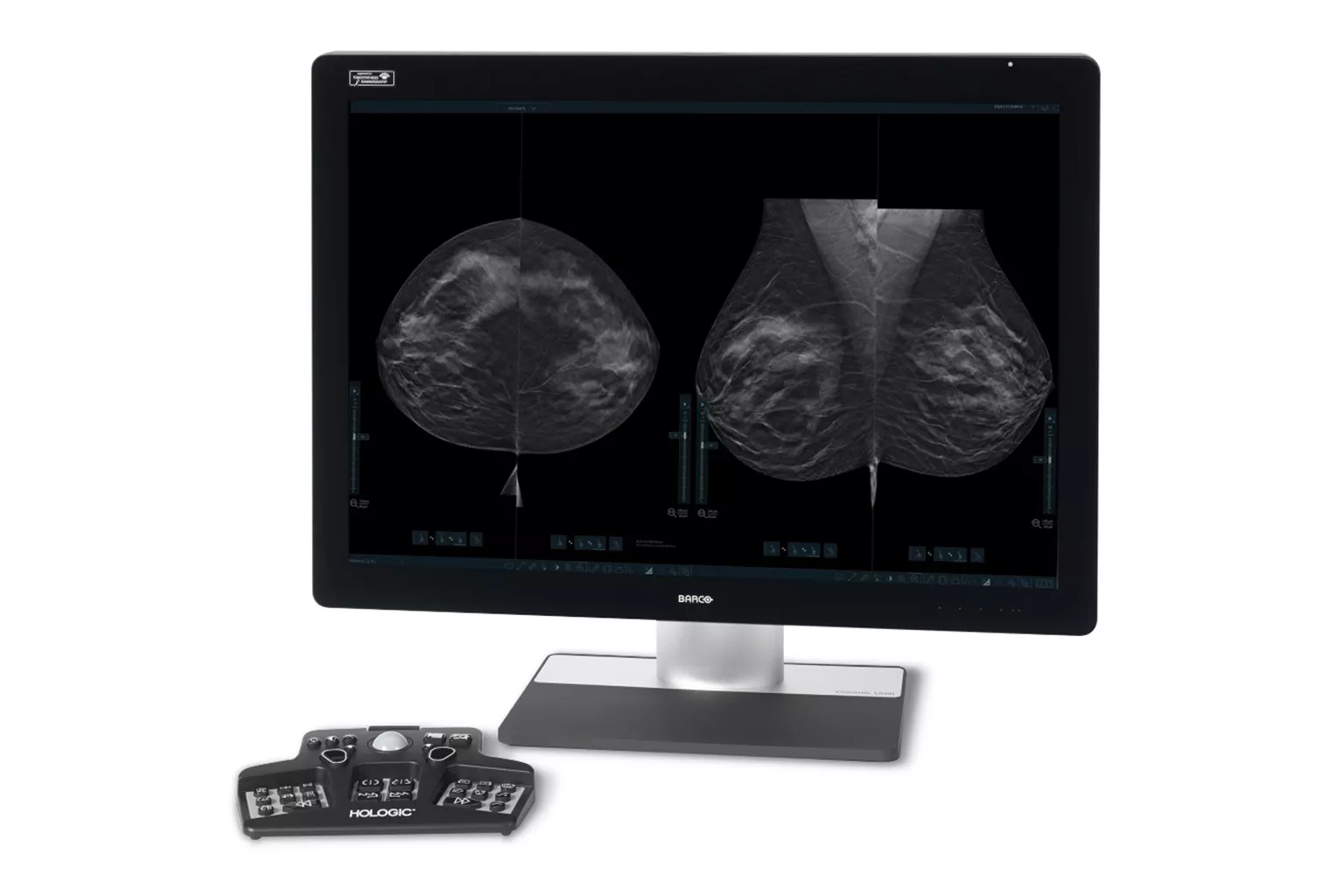
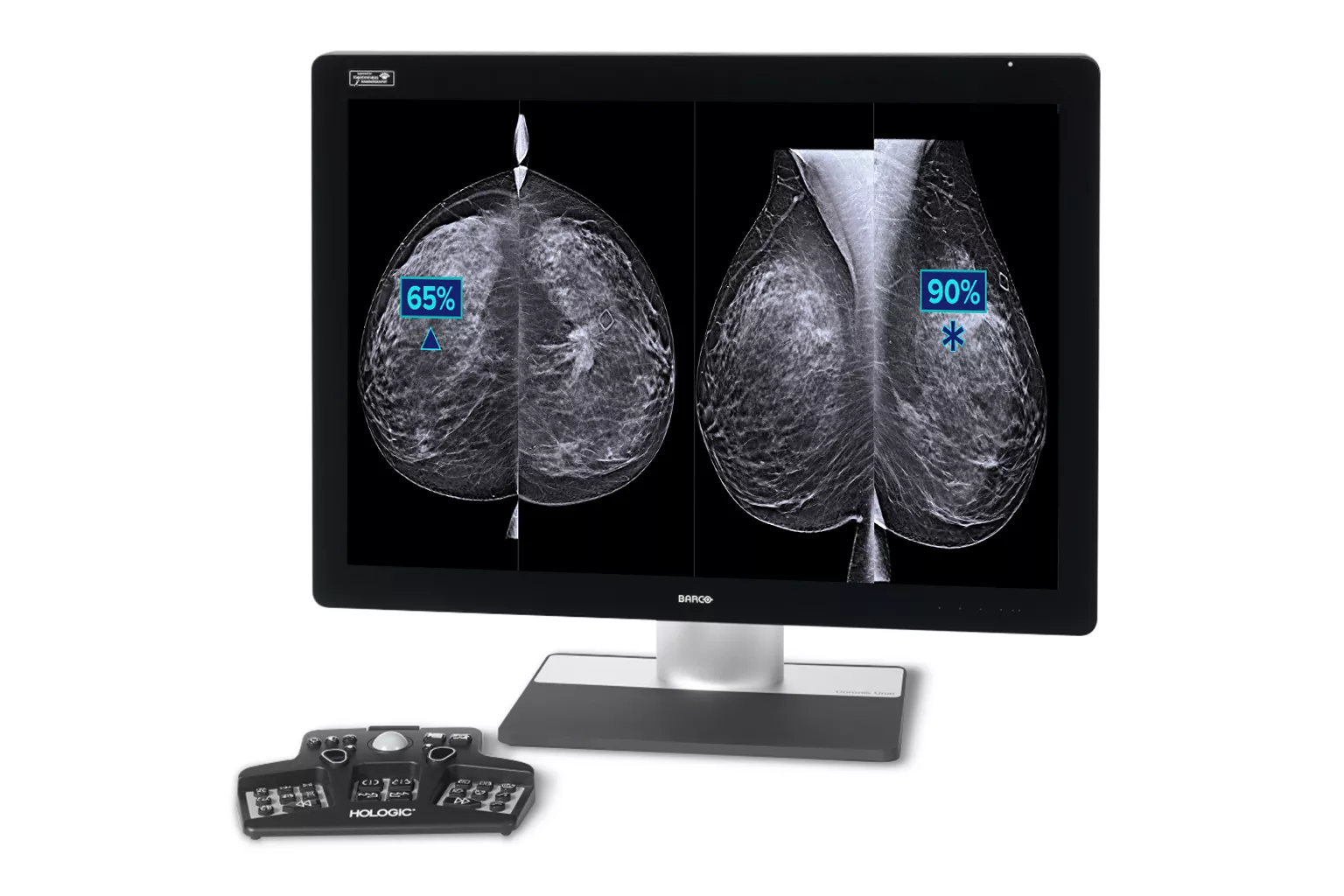
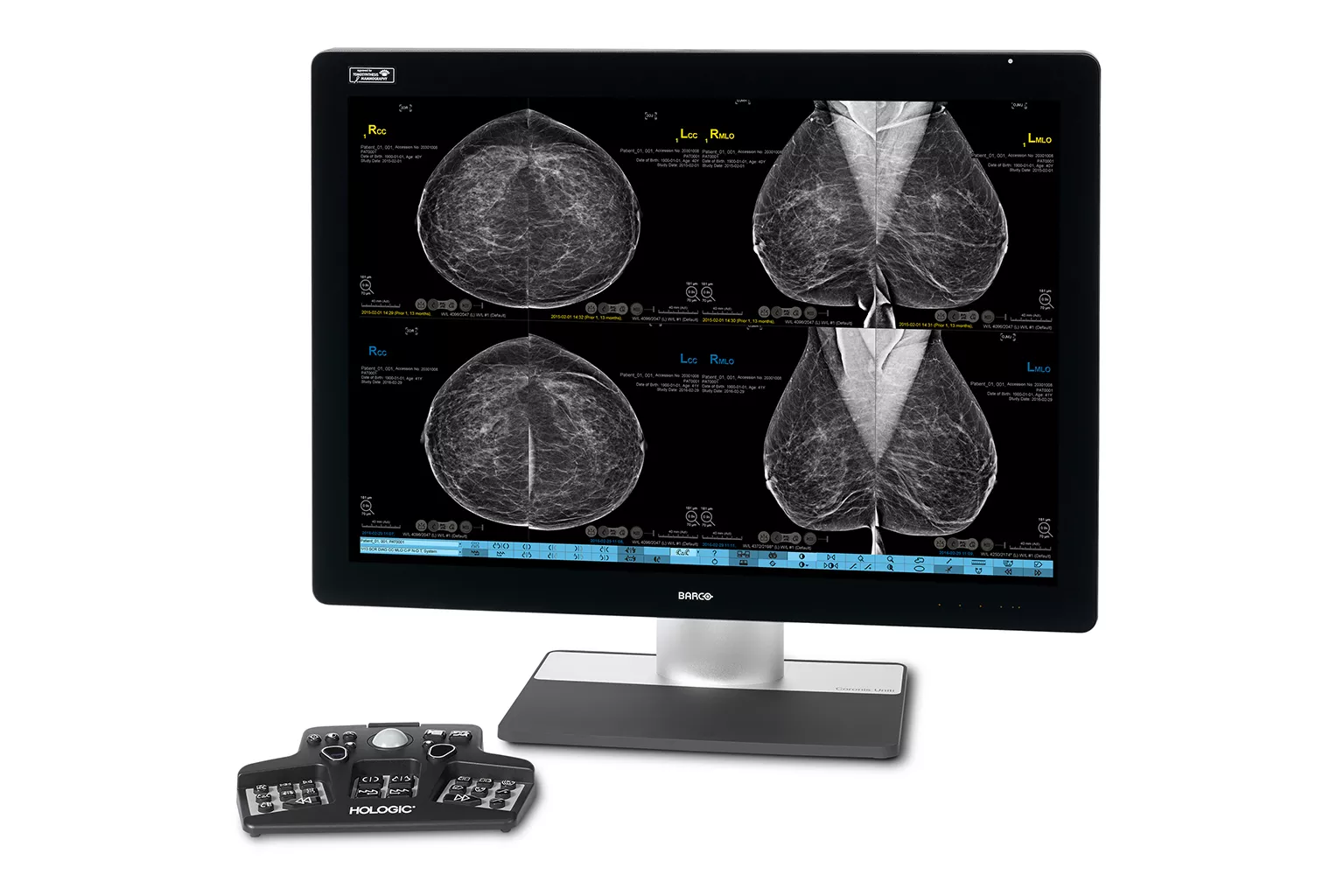
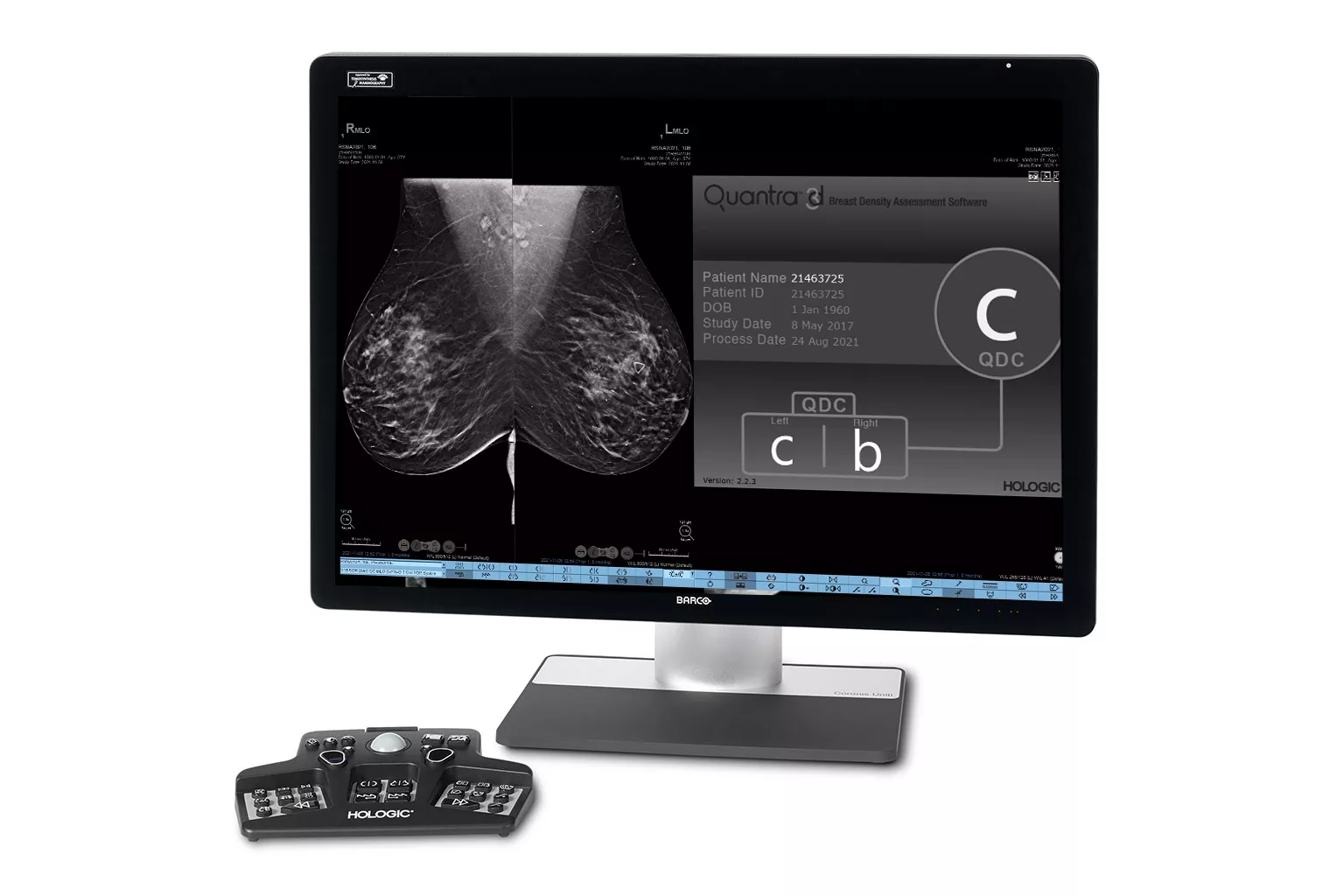
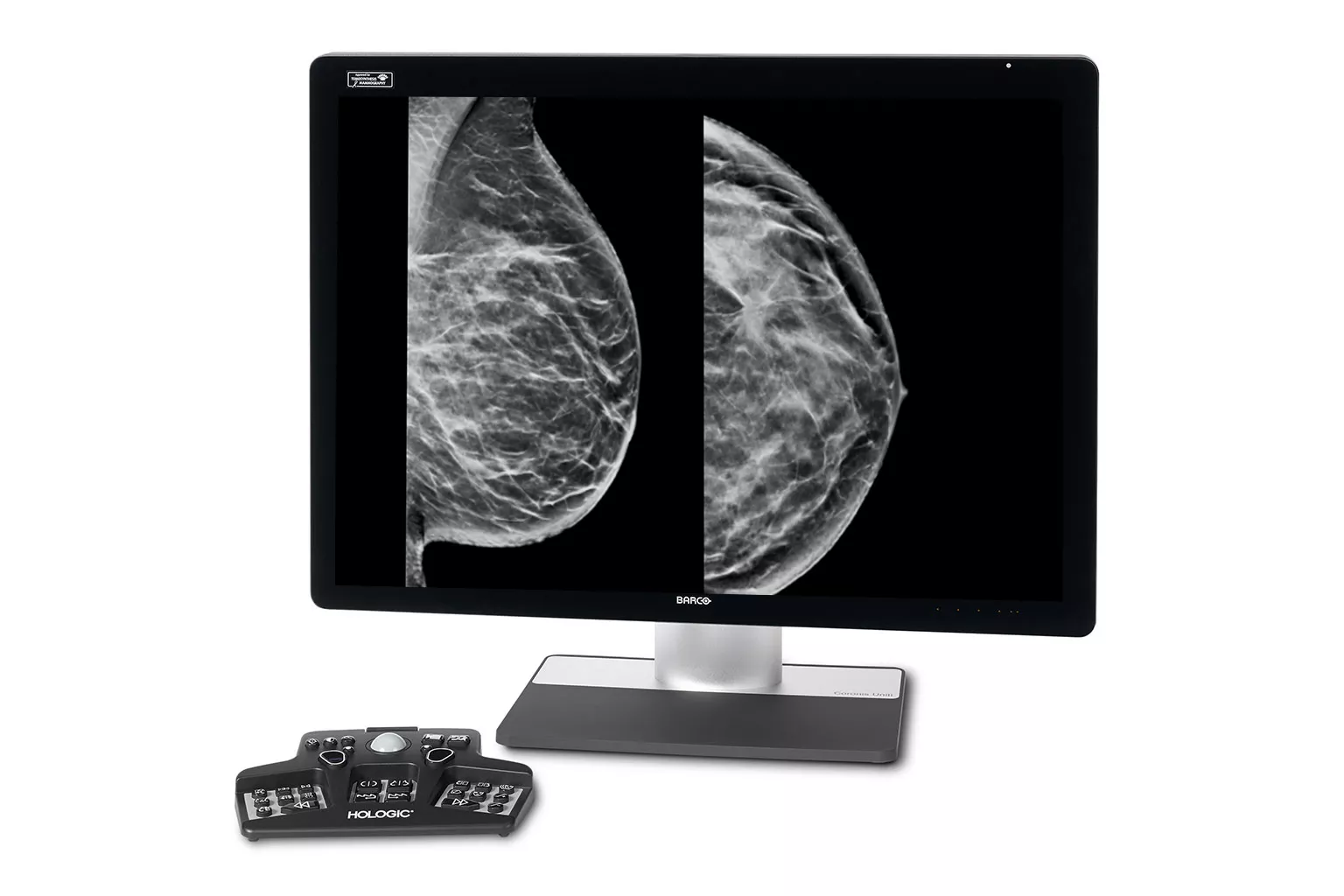
Image Analysis & AI
Exceptional image quality is important to us, which has inspired our goal to improve diagnostic clarity, and the vision to identify cancers quickly and accurately, even in dense breasts. All of our systems are built with patient compassion in mind, from shorter compression times, to features designed to maximize comfort.2, 3
Our breast image analysis technology is designed with cutting-edge science and powerful algorithms to accelerate discovery of suspicious lesions, improve reading efficiency, and continually sharpen image reading precision.
Decades of Continuous Innovation
Our pioneering technology supports best-in-class detection and diagnostics,* delivered with speed and accuracy.1
3.7s
Scan Time, the industry’s fastest tomosynthesis scan² on the 3Dimensions™ Digital Mammography System**
1 Hour
Daily saving in image interpretation time3,4 with 3DQuorum™ Imaging Technology
Additional Cancer
+9% improvement in observed reading for approximately 1 additional cancer found for every 10 identified by a radiologist5
70 Micron
Clarity HD+® imaging technology provides the highest resolution 3D images in the industry6 to clearly see subtle lesions and fine calcifications.
Browse Innovative Solutions in Mammography and Breast Imaging Analysis
Visit Our Virtual Hospital
Browse our portfolio of Breast Health solutions in 3D. See how you can unlock the advantage of time across the entire Breast Continuum of Care.

* The only system to have received EUREF certification for both 2D and 3D.
** Compared to other standard models.
† When using 3D plus 2D exam
- U.S. Food and Drug Administration, Center for Device and Radiological Health. (2020, November 18). Genius AI Detection K201019 510(k) Summary. IN1FDA Clearance: K201019 *Based on analyses that do not control type I error and therefore cannot be generalized to specific comparisons outside this particular study. In this study: The average observed AUC was 0.825 (95% CI: 0.783, 0.867) with CAD and 0.794 (95% CI: 0.748, 0.840) without CAD. The difference in observed AUC was +0.031 (95% CI: 0.012, 0.051). The average observed reader sensitivity for cancer cases was 75.9% with CAD and 66.8% without CAD. The difference in observed sensitivity was +9.0% (99% CI: 6.0%, 12.1%). The average observed recall rate for non-cancer cases was 25.8% with CAD and 23.4% without CAD. The observed difference in negative recall rate was +2.4% (99% CI: 0.7%, 4.2%). The average observed case read-time was 52.0s with CAD and 46.3s without CAD. The observed difference in read-time was 5.7s (95% CI: 4.9s to 6.4s). www.accessdata.fda.gov/cdrh_docs/pdf20/K201019.pdf. FDA clearance K221449 Make #1 2 and add FDA 510(k) Clearance K230096.
- Rocha Garcia, A.M., Mera Fernandez, D. Breast tomosynthesis: State of the art. Radiologia. 2019;61(4):274-285.
- Mammomat Inspiration with PRIME Technology brochure retrieved on June 9, 2017 from https://static.healthcare. siemens.com/siemens_hwem-hwem_ssxa_websites-context root/wcm/idc/groups/public/@global/@imaging/@mammo/documents/download/mda1/nzez/~edisp/mammography_mammomat_inspiration_prime_mammography_screening_machine_product_brochure-feb-16-02678877.pdf
- Data on file: DHM-05051_002 MAN-02290 15. Lordache, R. (2015) Quality Control for SenoClaire (GE Breast Tomosynthesis) retrieved on June 9, 2017 from http://amos3.aapm.org/abstracts/pdf/97-26965-352470-110105-667065451.pdf
- U.S. Food and Drug Administration, Center for Device and Radiological Health. (2020, November 18). Genius AI Detection K201019 510(k) Summary. IN1FDA Clearance: K201019 *Based on analyses that do not control type I error and therefore cannot be generalized to specific comparisons outside this particular study. In this study: The average observed AUC was 0.825 (95% CI: 0.783, 0.867) with CAD and 0.794 (95% CI: 0.748, 0.840) without CAD. The difference in observed AUC was +0.031 (95% CI: 0.012, 0.051). The average observed reader sensitivity for cancer cases was 75.9% with CAD and 66.8% without CAD. The difference in observed sensitivity was +9.0% (99% CI: 6.0%, 12.1%). The average observed recall rate for non-cancer cases was 25.8% with CAD and 23.4% without CAD. The observed difference in negative recall rate was +2.4% (99% CI: 0.7%, 4.2%). The average observed case read-time was 52.0s with CAD and 46.3s without CAD. The observed difference in read-time was 5.7s (95% CI: 4.9s to 6.4s). www.accessdata.fda.gov/cdrh_docs/pdf20/K201019.pdf. FDA clearance K221449 Make #1 2 and add FDA 510(k) Clearance K230096.
- Data on file and from public sources, 2017
Related Portfolio & Solutions
Breast Health Continuum of Care
Time is precious when it comes to effective detection, diagnosis and treatment of breast cancer. We strive to save you time at every step along the Breast Health Continuum of Care ensuring more women have more time in better health.