Hologic's Commitment To Delivering Essential COVID-19 Testing
Accurate and fully automated testing is critical in the fight against the coronavirus disease (COVID-19) – and key to quickly identifying who is infected and subsequently helping alleviate the spread of this novel virus.
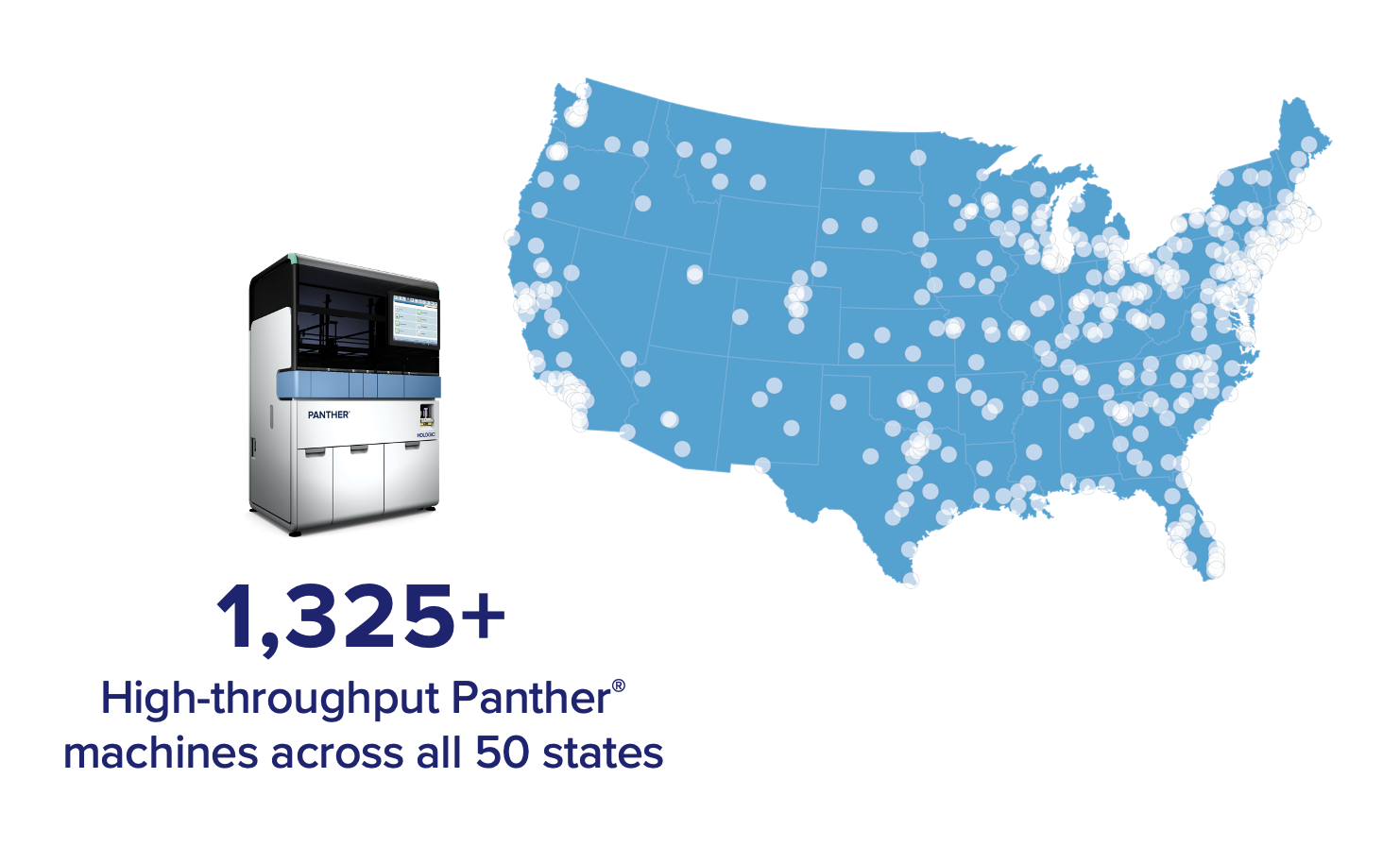
Hologic is proud to offer COVID-19 testing across all 50 states with multiple sample types. Hologic is committed to delivering more than 10 million new COVID-19 tests to our laboratory partners per month.
Hologic COVID-19 Tests
Hologic COVID-19 tests should be ordered for the detection of coronavirus in individuals who are suspected of having COVID-19.* Download our specimen collection guides for more information:
- Nasopharyngeal (NP) swab
- Nasal swab
- Patient-collected nasal swab
- Throat swab
- Nasal wash/aspirate†
- Lower respiratory tract specimen‡
- Nasal swab (VTM)
- Patient-collected nasal swab (VTM)
- Throat swab (VTM)
*Specimens should be collected with the appropriate infection control precautions. Use appropriate personal protective equipment when collecting and handling specimens from individuals suspected of having COVID-19.
† Nasal wash/ aspirate sample is indicated for Aptima® SARS-CoV-2 assay.
‡ Lower respiratory tract specimen sample is indicated for Panther Fusion® SARS-CoV-2 assay.
Test Results
A positive test result for COVID-19 indicates that RNA from SARS-CoV-2 was detected, and the patient is infected with the virus and presumed to be contagious. Laboratory test results should always be considered in the context of clinical observations and epidemiological data in making a final diagnosis and patient management decisions. Patient management should follow current CDC guidelines.
The Hologic COVID-19 tests have been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks to patients could include the following: a recommendation for isolation of the patient, monitoring of household or other close contacts for symptoms, patient isolation that might limit contact with family or friends and may increase contact with other COVID-19 patients, limits in the ability to work, the delayed diagnosis and treatment for the true infection causing the symptoms, unnecessary prescription of a treatment or therapy, or other unintended adverse effects.
What does it mean if the specimen tests negative for the virus that causes COVID-19?
A negative test result for this test means that SARS-CoV-2 RNA was not present in the specimen above the limit of detection. However, a negative result does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.
When diagnostic testing is negative, the possibility of a false negative result should be considered in the context of a patient’s recent exposures and the presence of clinical signs and symptoms consistent with COVID-19. The possibility of a false negative result should especially be considered if the patient’s recent exposures or clinical presentation indicate that COVID-19 is likely, and diagnostic tests for other causes of illness (e.g., other respiratory illness) are negative. If COVID-19 is still suspected based on exposure history together with other clinical findings, re-testing should be considered by healthcare providers in consultation with public health authorities. Risks to a patient of a false negative include: delayed or lack of supportive treatment, lack of monitoring of infected individuals and their household or other close contacts for symptoms resulting in increased risk of spread of COVID-19 within the community, or other unintended adverse events.
The Hologic COVID-19 tests are only authorized for use in laboratories in the United States certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests.
The United States (U.S.) FDA has made the Hologic COVID-19 tests available under an emergency access mechanism called an Emergency Use Authorization (EUA).
Our Public Health Legacy
Hologic’s contributions to the fight against COVID-19 extend our decades of innovation toward improving public health through early detection.
In 1997, for example, we developed a technology for identifying HIV in blood samples. Today, about 75 percent of the United States’ supply of donated blood is screened for HIV using that breakthrough method.
Hologic’s HIV detection efforts have expanded to include two molecular assays that run on the Panther system. These tests enable us to serve as a growing source of HIV screening in sub-Saharan Africa, where more than two-thirds of the world’s HIV infections occur.
Other Hologic assays identify pathogens that collectively cause suffering for billions of people worldwide each year, including cervical cancer, sexually transmitted infections and influenza.

