Vaginitis and STIs: A Complicated Web of Co-infections
The accuracy of NAAT testing can help counter the risk of vaginitis and STI co-infection
Vaginitis and sexually transmitted infections (STIs) are common infections that are generally well-known in the public health sphere, but what is less known is the correlation between them and the serious health implications of co-infection if left untreated.
Vaginitis (inflammation or infection of the vagina) is estimated to impact 21 million women in the US each year.1,2 STIs, including chlamydia, gonorrhea, human papillomavirus (HPV), and human immunodeficiency virus (HIV), resulted in 26 million new infections in 2018, with half occurring in people between 15 and 24 years of age.3
Vaginitis is mainly caused by three different infections either alone or in combination: bacterial vaginosis (BV), Candida vaginitis (CV), and Trichomonas vaginalis (TV).4 If left untreated, vaginitis can negatively impact a woman’s sexual, physical, and mental health.5 BV and TV can potentially lead to pregnancy complications such as preterm birth and low birth weight.6 Candida infections can result in systemic infections.7 Similarly, untreated STIs can result in long-term health problems, such as pelvic inflammatory disease and ectopic pregnancies for women, and infertility for both men and women.3,8
High prevalence. Serious consequences. But what may be the most concerning issue with these infections is their relationship with each other, a relationship that may be underrecognized and underappreciated.9,10 One vaginitis study found that chlamydia and Mycoplasma genitalium (M. gen) were pervasive with BV infection, a 2-3 fold increase when BV is present.11 This same study also found that TV has significant ties to other STIs, with a 2-4 fold increase in rates of chlamydia, gonorrhea, and M. gen when TV is present.11 Due to these alarming levels of co-infection, the Centers for Disease Control and Prevention (CDC) recommends that women diagnosed with BV or TV should also be tested for STIs, such as chlamydia and gonorrhea.12,13
Because half of new STIs occur in people between 15 and 24 years of age, it is vital that we focus our efforts on accurate and comprehensive testing for this patient population, especially women whose reproductive and sexual health may be placed at risk.3,8
NAAT can help detect a larger number of infections
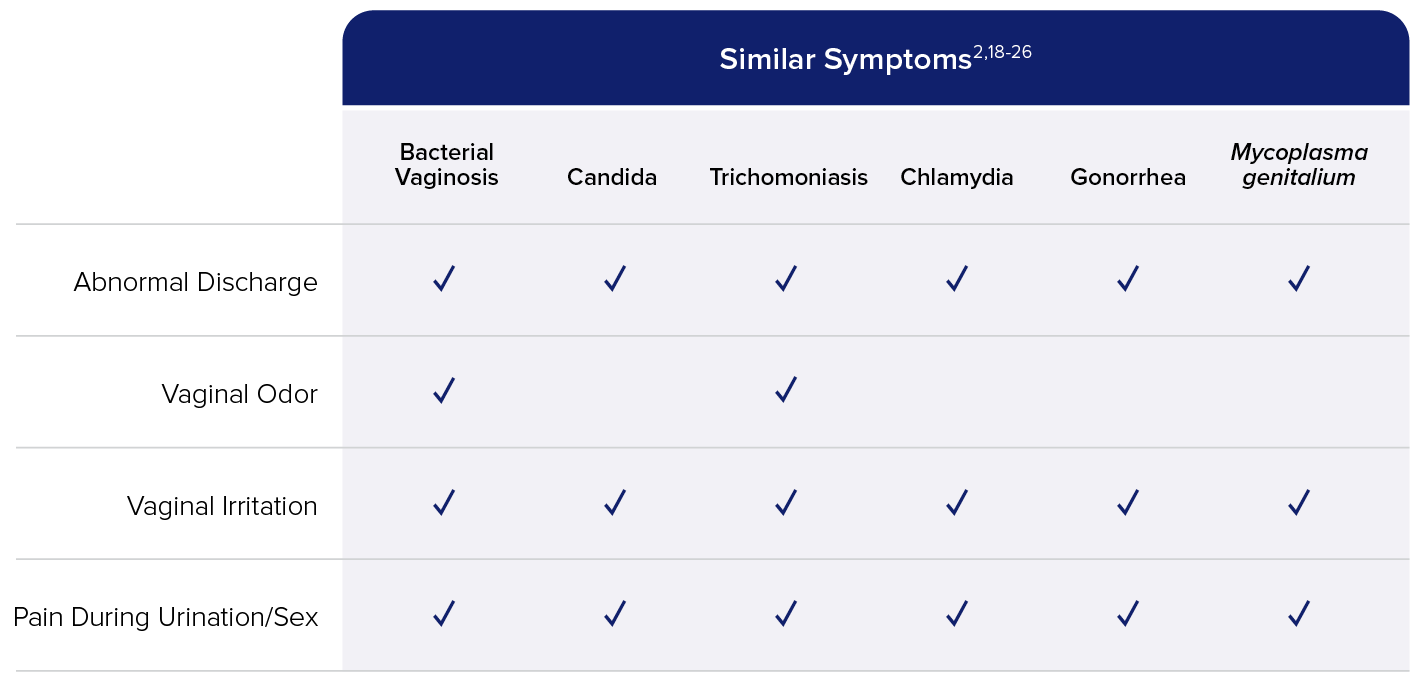
While vaginitis may be common, overlapping symptoms and mixed infections make diagnosis a challenge (see Figure). Symptoms can include any combination of the following: increased vaginal pH, abnormal vaginal discharge, odor, itching or burning, pain during urination or sexual intercourse, spotting or bleeding.14,15 Mixed infectious vaginitis cases, another complication, have recently begun to attract greater attention and are estimated to make up more than 20% of vaginitis cases.16 In fact, 37% of women diagnosed with BV are also infected with TV and/or Candida species.17 BV, the most common vaginal disorder, is of special concern with its high rate of causing vaginitis and increased susceptibility with STIs.3,4,6 Prospective studies have shown that BV can be a risk factor for HIV and other STIs such as HPV, herpes simplex virus (HSV), gonorrhea, and chlamydia.6
Mixed Infections and Overlapping Symptoms Make Diagnosis a Challenge17
37% of women with BV are also infected with TV and/or Candida species.17
Accurate testing is important to help protect a woman’s sexual and reproductive health. The importance of this is evident when you look at the interconnectivity of vaginitis, STIs and other health issues. The CDC recommends a nucleic acid amplification test (NAAT) as the testing method for numerous infections and patient populations, including symptomatic women with BV.6 Some healthcare facilities still rely on Amsel criteria, wet mount testing, and physical exams for BV testing, but these testing methods can result in misdiagnosis up to 60% of the time.27 NAAT allows for consideration of multiple targets indicative of vaginitis,28,29 and has the ability to detect mixed infections more frequently than either clinical evaluation or probe testing.30
Unlike other tests that only detect one BV agent, the Aptima® BV assay targets Lactobacillus (L. gasseri, L. crispatus, and L. jensenii), Gardnerella vaginalis, and Atopobium vaginae. The Aptima BV assay utilizes an advanced proprietary algorithm to report a simple qualitative positive or negative result.31 A recent study also demonstrated the assay’s ability to detect coinfection with STIs. Within this study, one in four women with a diagnosis of BV also had an STI, and the highest percentages for M. gen were in those positive for TV (15.1%) or BV (12.6%).11
Hologic’s diagnostics portfolio is a valuable tool for any busy laboratory that needs to produce objective, comprehensive and accurate results for a wide range of infections. The Aptima® CV/TV assay reports the Candida species group (C. albicans, C. tropicalis, C. parapsilosis, and C. dubliniensis), Candida glabrata and TV. The most prevalent, up to 90%, of CV cases is C. albicans, followed by C. glabrata as the second most prevalent.32 The Aptima CV/TV assay differentiates C. glabrata from the Candida species group providing easy-to-interpret qualitative results.33 This is important because CV (>20%) and TV (>15%) are estimated to cause >35% of all vaginitis cases.4
To further simplify and streamline sexual and vaginal health testing, the Aptima® Multitest Swab can detect up to seven infections and disease states from one vaginal swab (BV, Candida species, C. glabrata, TV, chlamydia, gonorrhea, and M. gen).31,33-36 Due to the high rates of vaginitis and STI co-infections,3,4,6 women diagnosed with BV by any method (clinic procedures or laboratory-based NAAT) should be tested for chlamydia, gonorrhea, and M. gen.6 To learn more about Hologic’s testing solutions and how they can help protect vaginal and sexual health, visit our Aptima® Vaginal Health page.

