Medical Device Safety Notification
BioZorb® 3D Bioabsorbable Marker
Dear Valued Hologic Customer,
Hologic Inc., would like to inform patients and health care providers about possible health risks reported with respect to the BioZorb Marker and BioZorb LP Marker (BioZorb Marker) implantable devices. Hologic has recently become aware of patient complaints which describe complications/adverse events that include pain, infection, rash, device migration, device erosion, seroma, discomfort, or other complications from feeling the device in the breast, and the need for additional medical treatment to remove the device. Hologic has notified and is working closely with FDA to evaluate all available information about the safety of BioZorb Markers and to address potential risks when used in breast tissue.
The BioZorb Marker is indicated for radiographic marking of sites in soft tissue. In addition, the Marker is indicated in situations where the soft tissue site needs to be marked for future medical procedures. The BioZorb Marker is an implantable radiographic marker not indicated to improve cosmetic outcomes after procedures or fill space in the tissue.
Our goal is first and always to provide high-quality products that enable our customers to provide safe, effective patient care to their patients. We are committed to providing you with the most accurate and up-to-date information on the use of the BioZorb marker and ensuring the ongoing safety and efficacy of our products.
This notification should be shared with all surgeons in your practice that have or will use the BioZorb Marker.
Recommendations for Patients
- If you experience any adverse events following the placement of your BioZorb Marker, please contact your health care provider.
- Discuss the benefits and possible risks of implantable breast tissue markers for breast cancer procedures with your health care provider.
- Report any problems or complications experienced following the placement of BioZorb Marker devices to Hologic at breasthealth.support@hologic.com and to the FDA’s MedWatch Adverse Event Reporting program.
Recommendations for Health Care Providers
- Be aware of reports of serious adverse events following the placement of the BioZorb Marker devices in breast tissue.
- Discuss the benefits and possible risks of BioZorb Marker devices with your patient.
- Continue to monitor patients who have an implanted BioZorb Marker for signs of any adverse events.
- BioZorb Marker and BioZorb LP Marker are not cleared to fill space in the tissue or to improve cosmetic outcomes after procedures.
- Inform your patient which device you plan to use, if you plan to implant a marking device during breast conservation surgery.
- Report any problems or complications experienced by patients following placement of the BioZorb Marker devices to Hologic. Complaints can be submitted to breasthealth.support@hologic.com and to the FDA’s MedWatch Adverse Event Reporting program.
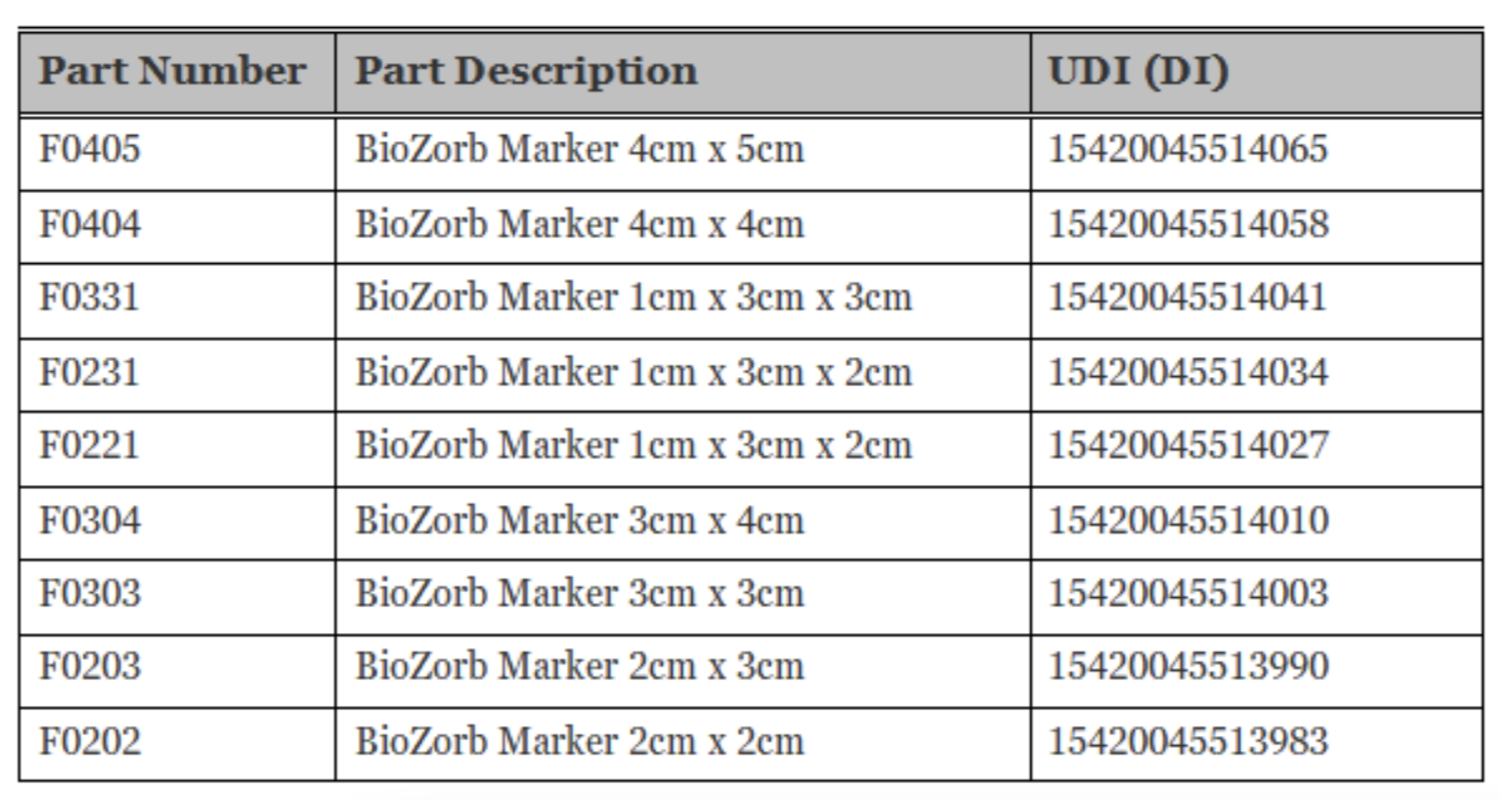
Unique Device Identifier (UDI)
The unique device identifier (UDI) helps identify individual medical devices sold in the United States from manufacturing through distribution to patient use. The UDI allows for more accurate reporting, reviewing, and analyzing of adverse event reports so that devices can be identified, and problems potentially corrected more quickly.
You can find the affected BioZorb Marker devices by checking the UDI table below. The UDI is a unique numeric or alphanumeric code that generally includes a Device Identifier (DI) that identifies the labeler and the specific version or model of a device, as well as a Production Identifier (PI) that identifies additional information, which may include lot number, serial number, expiration date, and manufactured date.
If you have any questions about this communication, please contact breasthealth.support@hologic.com.

