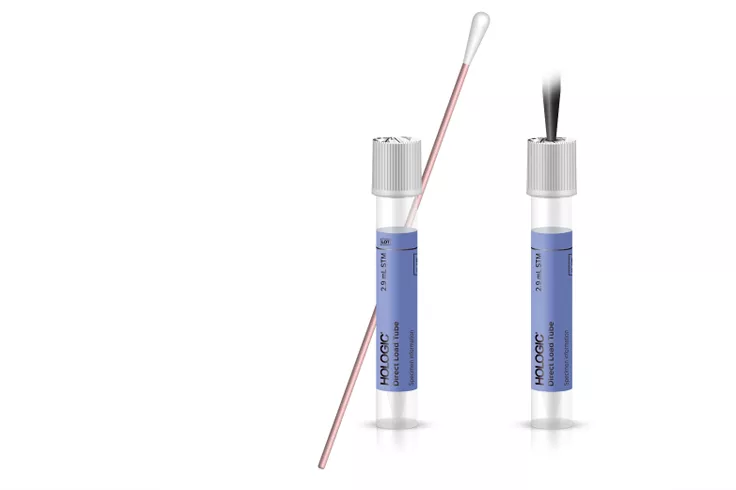
Direct Load Tube Collection Kit
No uncapping.
No specimen transfer.
Overview
Documents
Training
Transform Specimen Processing for COVID Testing
No uncapping and no specimen transfer. Expedite your SARS-CoV-2* workflow with a collection device that loads directly on the Panther® system. The Direct Load Tube Collection Kit is authorized for use with the Aptima® SARS-CoV-2 and Aptima SARS-CoV-2/Flu assays.
Deliver Results Sooner

Load directly into the Panther System.
- Directly load alongside specimen types for other assays with true random and continuous access.
- Non-hazardous lysis buffer reduces risk by inactivating common respiratory viruses, minimizing biosafety needs.
- Penetrable cap eliminates uncapping and recapping of tube.
Traditional 8-Step Respiratory Specimen Workflow
1
Uncap Collection Tube
2
Uncap Transfer Tube
3
Transfer Primary Specimen
4
Recap Transfer Tube
5
Recap Collection Tube
6
Mix Transfer Tube
7
Uncap Transfer Tube
8
Load Onto System
Reduce Labor/Costs/Risk with Fewer Steps
- Eliminate manual uncapping and recapping.
- One collection kit for nasal and OP swabs.
- Remove manual specimen transfer steps.
- Inactivates common respiratory viruses.
- Reduce human error and repetitive motion injuries.
- Guanidine free, non-toxic media.
- Reduce costs for additional consumables.
- Penetrable cap serves as additional cross-contamination barrier.


