Panther Fusion® GI Bacterial Assay
Flexible testing with sensitive detection of the most common bacterial causes of acute diarrhea — diagnostic insight faster.

Overview
Documents
Training
Molecular Insights for Gastrointestinal (GI) Care
Infectious gastroenteritis can be caused by a variety of bacterial, viral and parasitic organisms. Rapid and accurate diagnostic testing is essential to identify the infectious agent and guide the right treatment for patients.¹
The Panther Fusion® GI Bacterial Assay supports informed clinical decision-making with differential diagnosis of the most common bacterial causes of acute diarrhea while seamlessly integrating into existing laboratory workflows.
Superior Workflow2
Valid rate of 97.8% after initial test and a 99.6% valid rate after retest,2 on a system with a large sample capacity, high throughput and minimal hands-on time.
Sensitive and Specific2
In a clinical trial evaluating over 1700 samples, The Panther Fusion GI Bacterial Assay showed a positive percent agreement (PPA) of more than 97% for all targets.2
Customizable Flexible Panel
Deliver only the results needed with customizable testing that simplifies diagnostic stewardship.
Just a few Bacteria Account for Most Foodborne Illness in the United States3
Other than Norovirus, Campylobacter, Salmonella and STEC are some of the leading causes of foodborne illness in the United States.
Salmonella spp.
1.28M cases per year
Among foodborne illnesses, Salmonella is the leading cause of death.3
Campylobacter spp.
1.87M cases per year
Campylobacter is the most common bacterial cause of diarrheal illness in the United States.3
Shigella spp.
450,000 infections
Antimicrobial-resistant Shigella infections result in an estimated $93 million in direct medical costs.⁵
E. coli (STEC)
265,000 cases per year
STEC O157 causes about 36% of these cases, and non-O157 serogroups cause the rest of the cases.4
More than gut instinct. Performance you can count on.
The Panther Fusion® GI Bacterial Assay delivers rapid Multiplex real-time PCR detection of the most common bacterial causes of acute gastroenteritis. Deliver results that can enhance patient care and streamline laboratory workflows without adding needless complexity.
Bacterial GI targets
- Salmonella spp.
- Shigella spp./EIEC
- Campylobacter spp.
- Shiga toxin (STEC)
Part of a broad portfolio of molecular tests on a single platform, The Panther Fusion® GI Bacterial Assay can help support informed clinical decision-making and simplify stewardship programs.
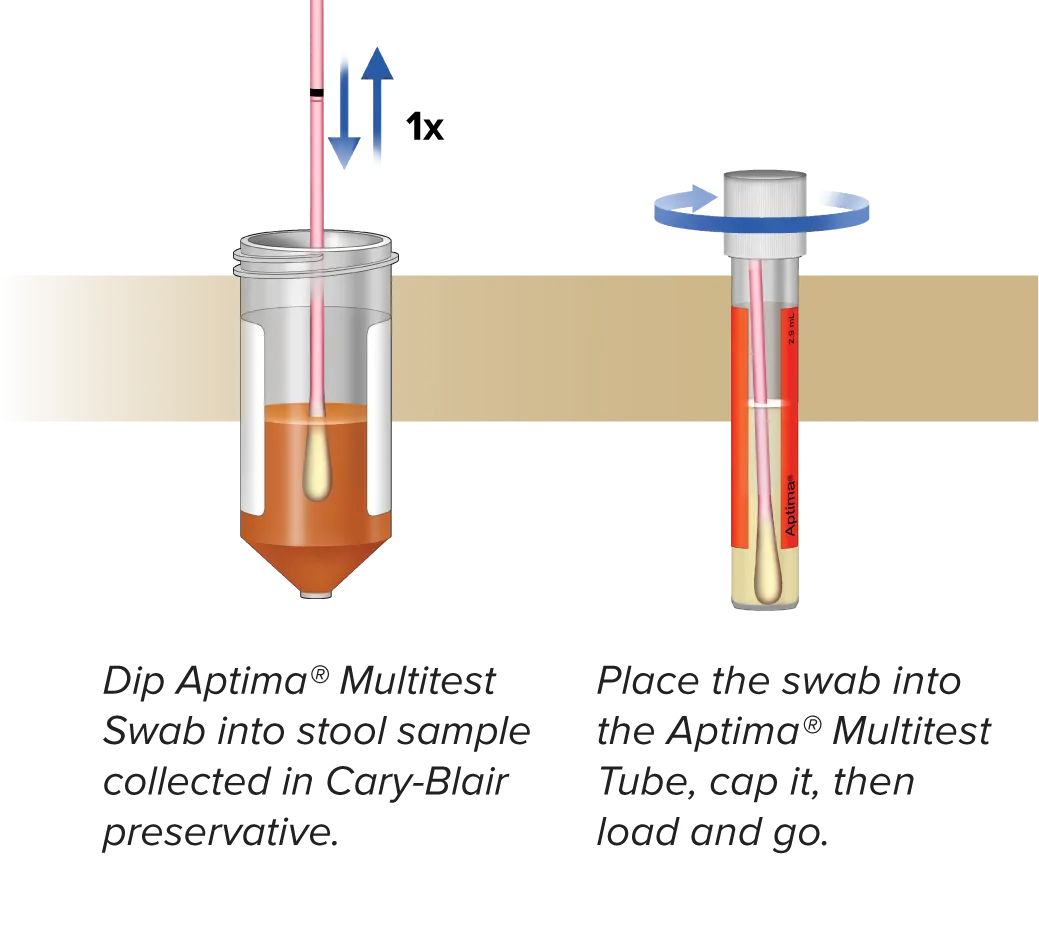
Simple pre-analytical processing with Aptima® Multitest Tubes lets you run just one GI panel at a time or all at once without adding extra steps.
Innovation never rests, and neither do we.
Our GI portfolio is under development to bring the diagnostic power of Hologic to a wide range of gastrointestinal illnesses.
Panther Fusion® GI Viral Assay*
- Norovirus
- Adenovirus
- Rotavirus
- Sapovirus/Astrovirus
Panther Fusion® GI Parasite Assay*
- Giardia lamblia
- Cryptosporidium spp.
- Entamoeba histolytica
- Cyclospora cayetanensis
Panther Fusion® GI C. difficile Assay*
- Toxigenic C. difficile
- Toxigenic hypervirulent C. difficile PCR ribotype 027 (NAP1/B1/027)
* In development and not for sale

Unleash the power of the Panther Fusion® System
- Unleash the Power of the Panther® System. High-throughput, random-access workflows give your lab both the capability and flexibility to deliver.
- Random & Continuous Access. No more batching; load samples with different test orders as they arrive. Run multiple assays simultaneously.
- Rapid Turnaround Time and Flexible Testing. Automation speeds up processing and allows you to prioritize tasks without delay.
- Simple pre-analytical processing. A single sample can be tested with any combination of Panther Fusion GI Assays — no additional hands-on steps required.