Newsroom
Find new product details, partnership news, press tools and more.
Press inquiries can be directed to media@hologic.com.

Behind the Science of Sure: Redefining What’s Possible in Cancer Detection with Artificial Intelligence
Topics
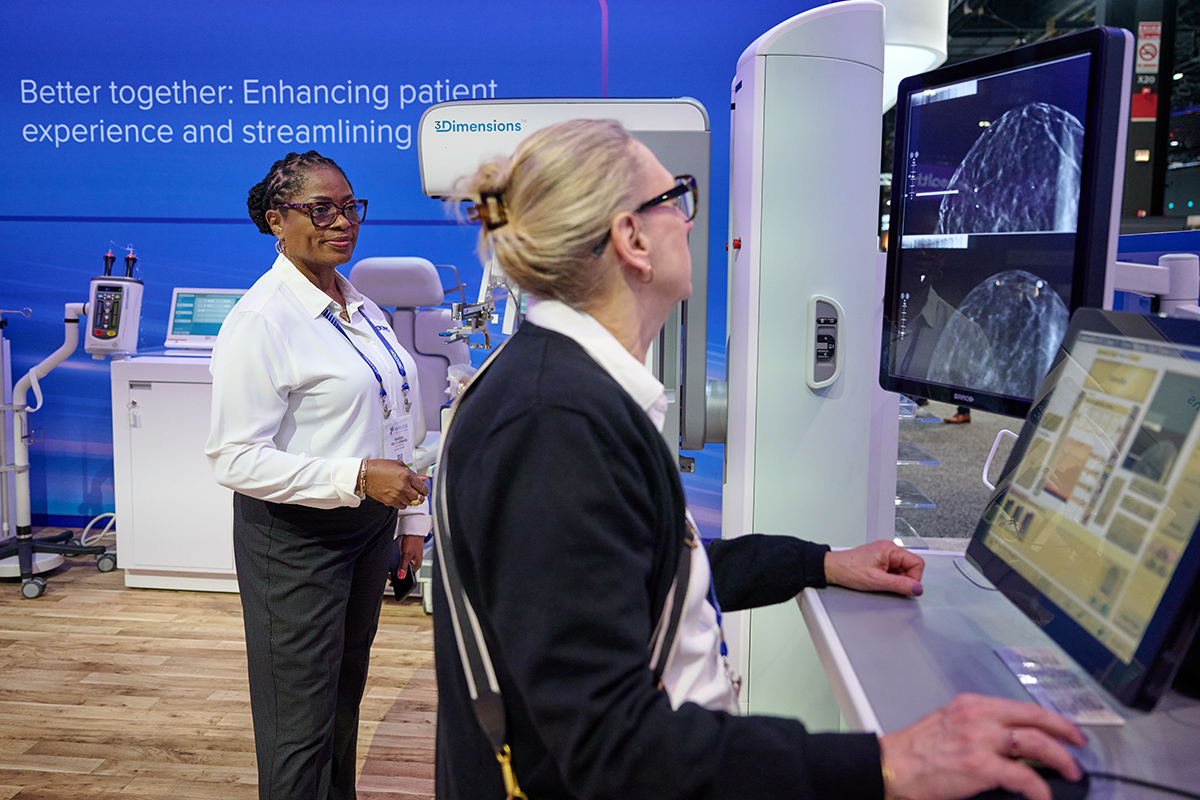
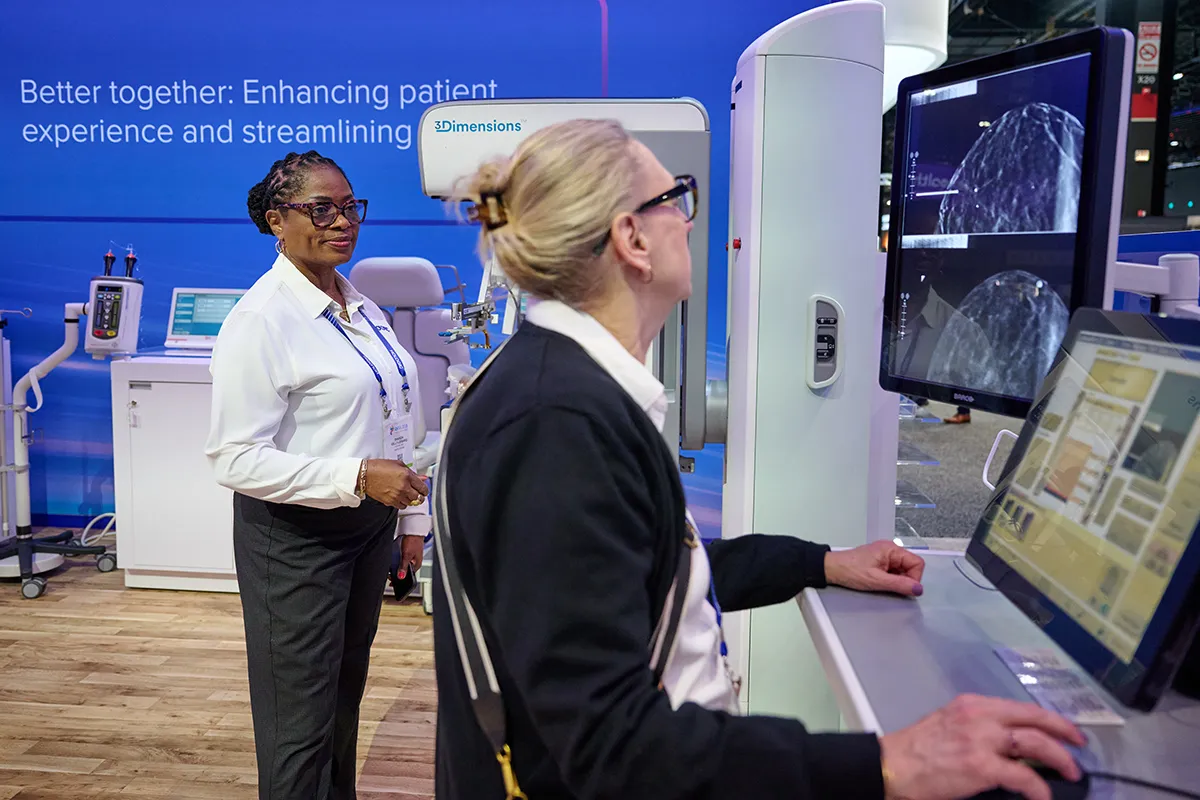
Global Reach, Personal Touch: AI’s Potential to Transform Breast Cancer Screening for Patients Everywhere

From 3D to AI and Beyond, How Hologic Continues to Shape the Future of Mammography


At the Intersection of Business and Science: Making Sustainability Part of Everyone’s Job


Experts Sound Alarm on Gender Gaps in Medical Reimbursement Rates and Their Impact on Women’s Health

Our Commitment to Sustainability
Shingo Prize: A World-Class Culture of Excellence
Hologic Costa Rica earned the prestigious Shingo Prize, an assessment of how a company's culture drives world-class results and the guiding principles of the Shingo Model™.
Topics

Professional Basketball Player Erica Wheeler and Hologic Team Up to Champion Cervical Cancer Screening
Press Inquiries
Global Reach, Personal Touch: AI’s Potential to Transform Breast Cancer Screening for Patients Everywhere
From helping radiologists detect breast cancer earlier to streamlining mammography workflows, AI is rapidly transforming the field of medical imaging and breast health. Liz Asai, Senior Director, Global Head of AI and Connected Health at Hologic, shares how these technologies are shaping the future of patient care.

At the Intersection of Business and Science: Making Sustainability Part of Everyone’s Job
The health of our loved ones and our communities are inseparable from the health of our planet. That’s why sustainability is not just a corporate initiative at Hologic; it is woven into what we do and how we do it. Sharon Vidal, Global Head of Sustainability shares how we’re rethinking product design to prioritize waste reduction, improving energy and water efficiency to reduce our footprint and many other efforts to drive progress.
Putting Women’s Health at the Heart of the Global Agenda
How can we close gaps in women’s health? Takeaways from a recent panel discussion with global leaders led by Mia Keeys, DrPH, MA, Director of Global Health and Innovation, point to three key themes.


With STIs on the Rise, Dr. Kyle Bukowski Shares His Insights on Screening and Why We Need to Break the Silence on This Critical Topic
While STI rates climb, the number of people being tested for them remains low, especially since most of these infections don’t cause any symptoms. According to Hologic’s Global Women’s Health Index, just 10% of women reported being tested for any type of STI in the past year. Untreated STIs can lead to a condition called pelvic inflammatory disease, which can cause chronic pain and infertility in women.
How Real-World Evidence is Shaping the Future of Healthcare Innovation
Regulatory science helps ensure modern, safe and effective diagnostic and medical technology solutions make it to market as efficiently as possible. Jeff Hergesheimer, Senior Director of Regulatory Affairs at Hologic, highlights the pioneering use of real-world evidence to continue to advance this field, fuel healthcare innovation and help improve patient outcomes.

“The definition of women's health has been so narrow for so long. It’s upon us as an industry to help shift and change that definition to be more inclusive of the whole woman and not just gender-specific organs.”
Jessica Hameline
Corporate Vice President, Strategy & Business Development
Women are struggling more to meet their basic needs today than at any point in almost two decades.
“With breast cancer we know that the earlier you find it, the better the chances of survival — and with AI algorithms advancing all the time, this technology’s going to continue to revolutionize healthcare in extraordinary ways.”
Ashwini Kshirsagar
Director of R&D, Product Owner, AI
"We’re seeing a steady shift toward better tools and technologies for women’s health.”
Dr. Kyle Bukowski
Practicing OB-GYN and Medical Director at Hologic
About 200 million more women
are worried and sad today than four years ago.
Championing Women’s Health Globally
Women's Empowerment by the Numbers
When women and girls don’t have access to healthcare, we can’t make meaningful progress on ending poverty because health impacts every other area of a woman’s life.” -Mia Keeys, Director of Global Health and Innovation, at the 2025 Global Citizen NOW event, where she discussed takeaways from the Hologic Global Women’s Health Index.
Our story in numbers
36
Countries
1985
Hologic is founded
4,100+
Patents
Our Commitment
For four decades, we have transformed early detection, championed preventive care and fueled innovative surgical solutions – and we work every day to improve the health and lives of all women, everywhere, every day.
Didn’t find what you’re looking for?
Let us know what you need and we will try to help you find it.







