Overview
Documents
Training
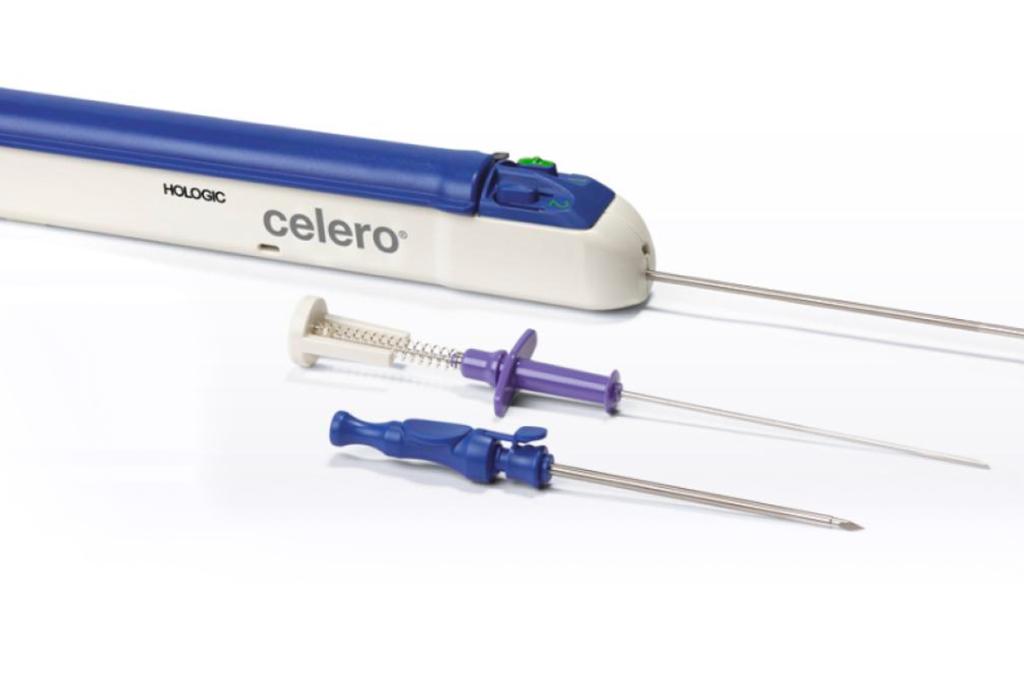
Untethered freedom. Designed for more control, consistent cores, and better access.
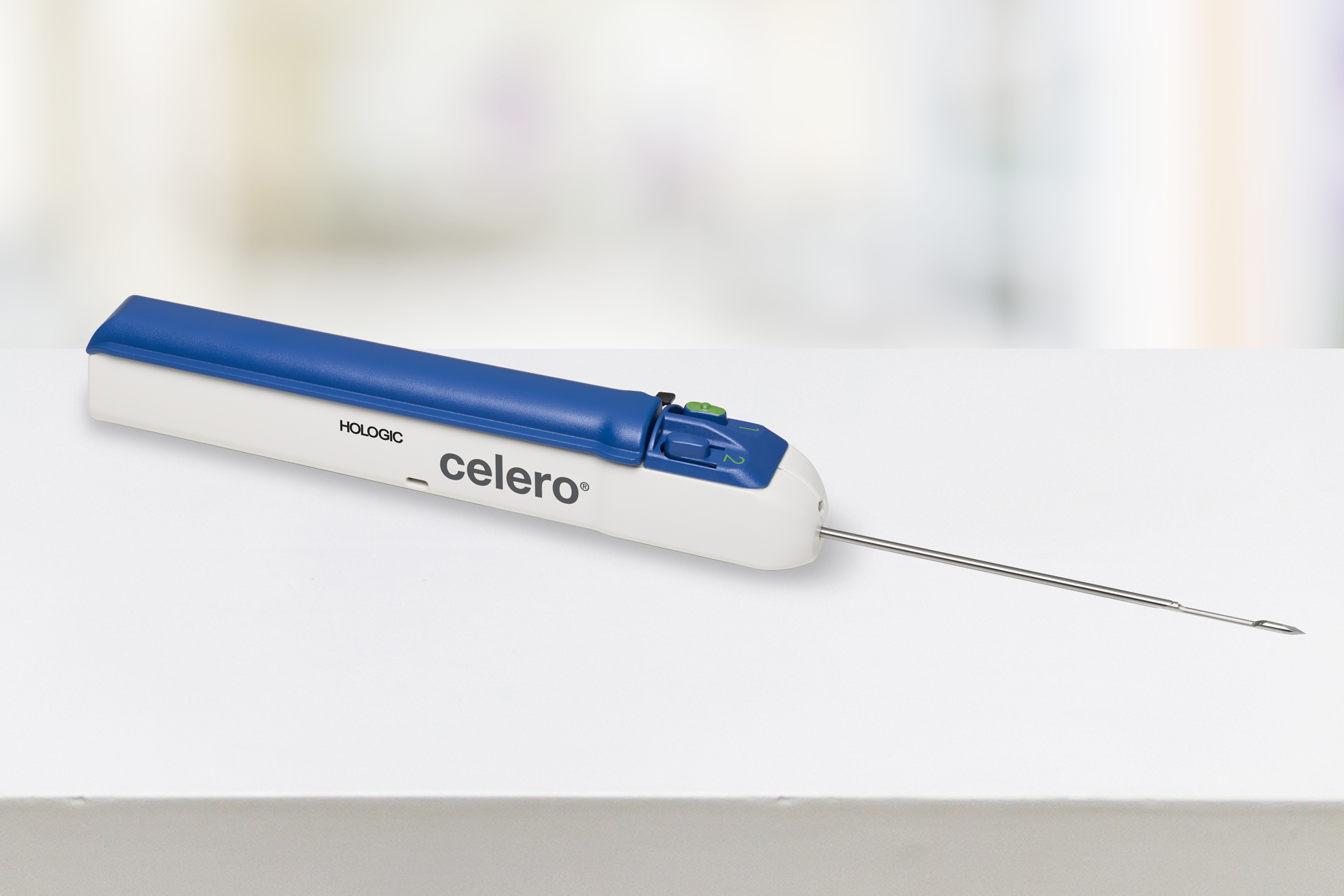
The Celero biopsy device is the first spring-loaded core device with integrated vacuum technology that offers untethered freedom during ultrasound breast biopsy. The Celero biopsy device is designed for more control, consistent cores, and better access.
More Control
Confidently approach challenging lesions.
11º trocar tip for smooth penetration
10mm dead space
Option to prefire for no throw insertion and clearly visible aperture
Consistent Cores
Ensure large samples and consistent cores with the first spring-loaded core device with integrated vacuum technology.2
Better Access
Make fewer insertions for accurate diagnosis with cores more than two times larger than other 14G SLC devices.1
See for Yourself
With the Celero biopsy device, you can achieve your goals with fewer samples and large-quality cores. That means fewer needle insertions and a more compassionate breast biopsy for your patient versus traditional spring-loaded core devices.
Product gallery
Specifications
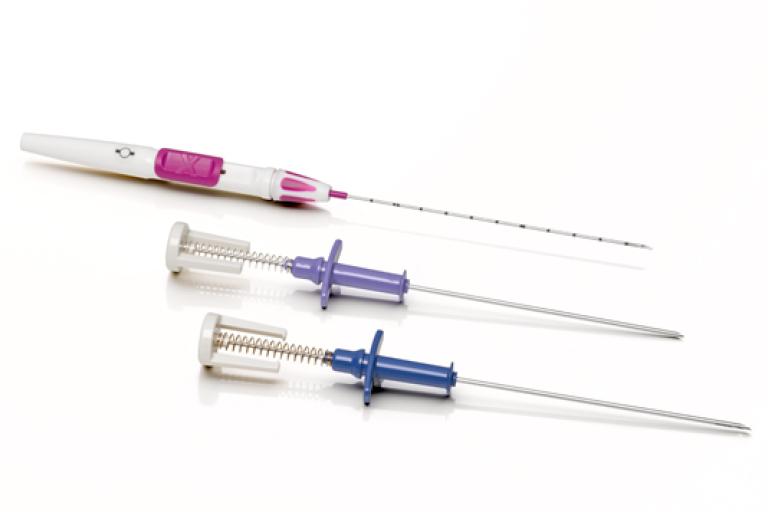
With multiple 9g and 12g needle options in standard and petite configurations; superficial, chest wall, and retroareolar lesions are easily handled by choosing the right needle for the task.
- 12 gauge
- 25mm throw
- 22mm aperture
- 10mm dead space
- 12-gauge introducer.
- Features a luer fitting for a secure fit and smooth transition during marker deployment.
- A quick-release thumb latch that allows for anesthetic delivery with aspiration option.