Overview
Documents
Training
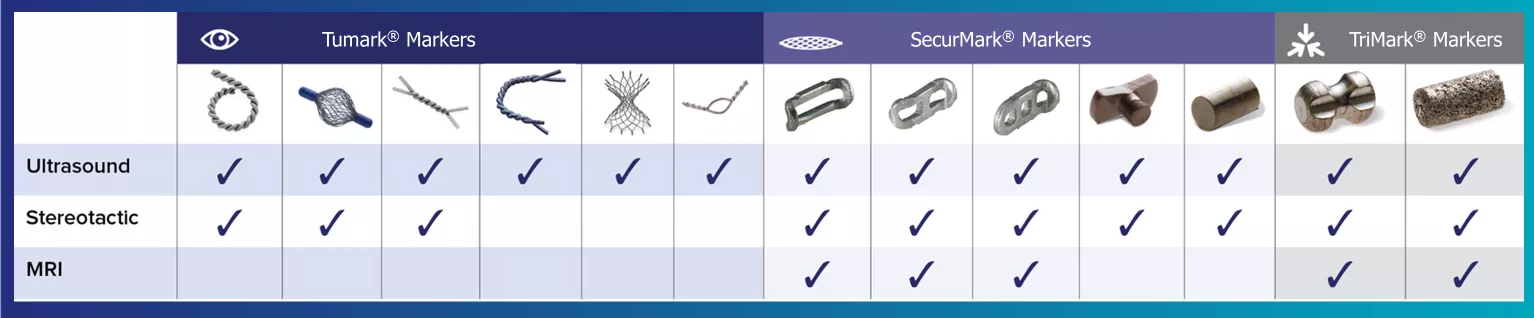
A variety of shapes and options for every modality.
Hologic's range of biopsy site markers come in multiple shapes, gauges and lengths, empowering physicians to individualize each patient’s care. Our comprehensive selection of markers include options for use with stereotactic, ultrasound, and MRI guided biopsies. All markers come with an ergonomic and easy to use deployment device.
Tumark® Biopsy Site Markers
Visibility
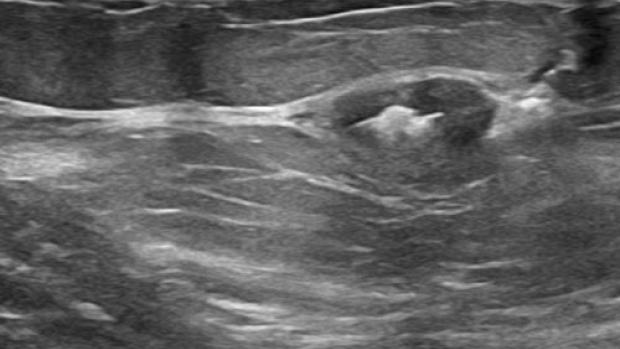
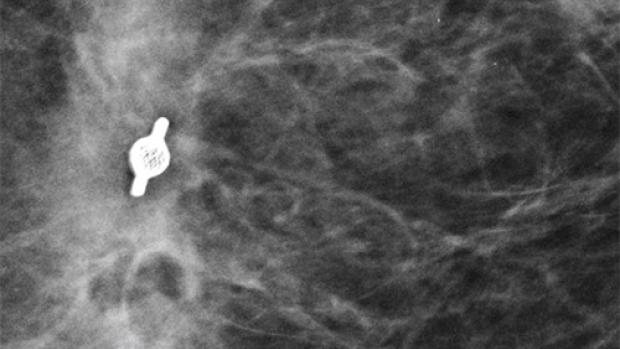
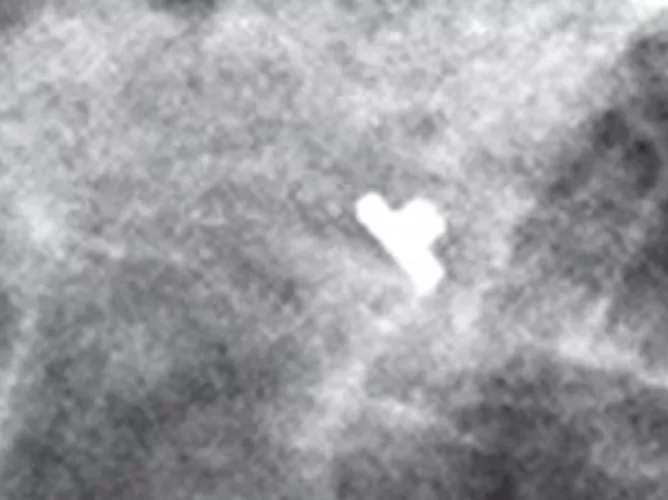
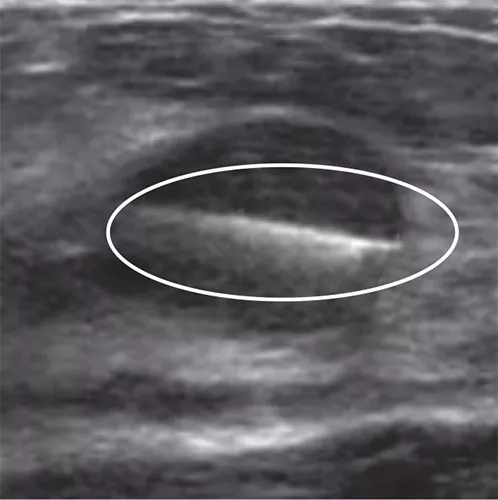
Tumark Professional Q and U markers are sandblasted nitinol, designed to provide outstanding visibility in ultrasound, and excellent visibility under mammography at deployment. In 85% of marker placements, physicians stated the ultrasound visibility was good to excellent upon deployment.2
Intelligent Design
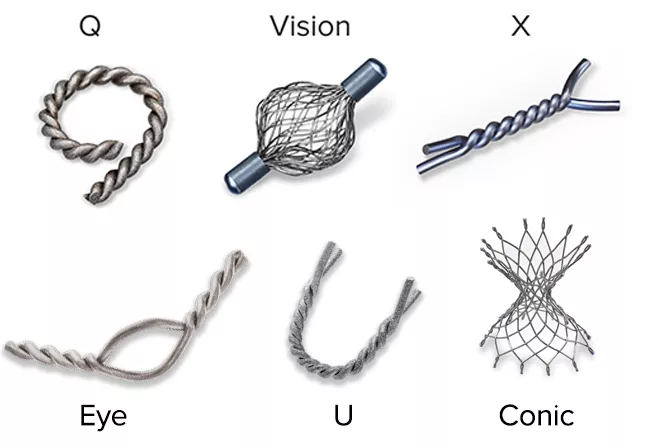
Intelligent design on all six distinct, easily identifiable shapes1 provides sound tissue fixation. Non-resorbing material expands into marker shape upon deployment to improve the stability of the anchor following a breast biopsy procedure.
Accurate Deployment
In the initial data collection study: 91% of markers placed under ultrasound did not migrate, as measured on the post-procedure mammogram.1 In 9 out of 10 cases the marker deployed accurately in ultrasound to the intended area.1
Single-handed Operation
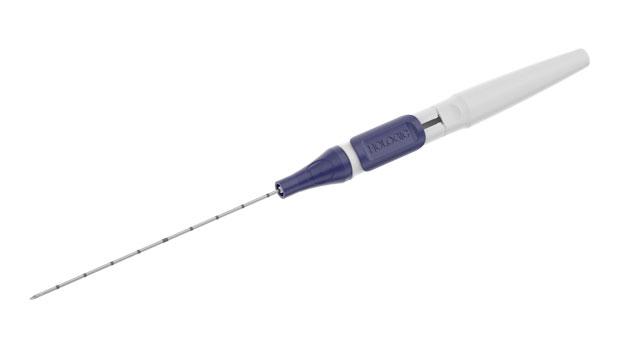
Ergonomic handle for single-handed operation. For all modalities, the deployment device includes either a sharp or blunt cannula and centimeter markings for depth orientation. In 99% of procedures completed, physicians agreed the handle was easy to use when deployed in ultrasound.1
Tumark Marker Shapes
Localize the biopsy site before surgery and after successful therapy with a marker designed for long-term visibility. Tumark breast biopsy marker options provide tissue fixation and visibility in ultrasound and stereotactic modalities.
Gallery
Our Tumark Offerings
85%
say ultrasound visibility was good to excellent upon deployment.1
92%
of markers deployed accurately to the intended area.1
99%
agreed the device was easy to use.1
SecurMark® Biopsy Site Marker
Enhance follow up ultrasound visualization three to four weeks post-biopsy with bio-absorbable suture-like netting.7
Minimize movement in the biopsy cavity with the bio-absorbable suture-like net. Mark multiple sites with five distinct bio-compatible titanium or stainless-steel permanent shapes.

Our SecurMark Offerings
TriMark® and CeleroMark™ Markers
Simple. Reliable.
Optimal for thin-breasted patients and superficial lesions. Smooth marker deployment to the biopsy site with rigid end deploy beveled tip.

A Shape for Any Case