Overview
Documents
Training
Introducing LOCalizer, the wire-free breast lesion system from Hologic.
The LOCalizer wire-free guidance system is designed to mark and guide to non-palpable breast lesions using a miniature radiofrequency identification (RFID) Tag. Each Tag has a unique identification number that is displayed on the reader and can be placed in the breast any time prior to or on the day of surgery.
Maximize schedule flexibility without the hassle of complex regulations associated with radioactive seeds.

LOCalizer System Features:
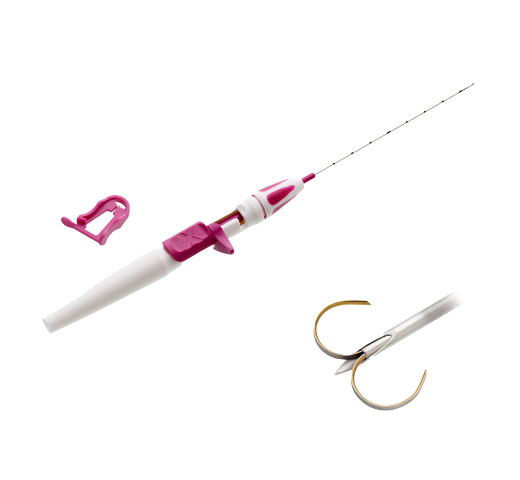
Miniature RFID tag with unique ID # pre-loaded in a needle applicator
Single-use surgical probe with small, ~8mm tip
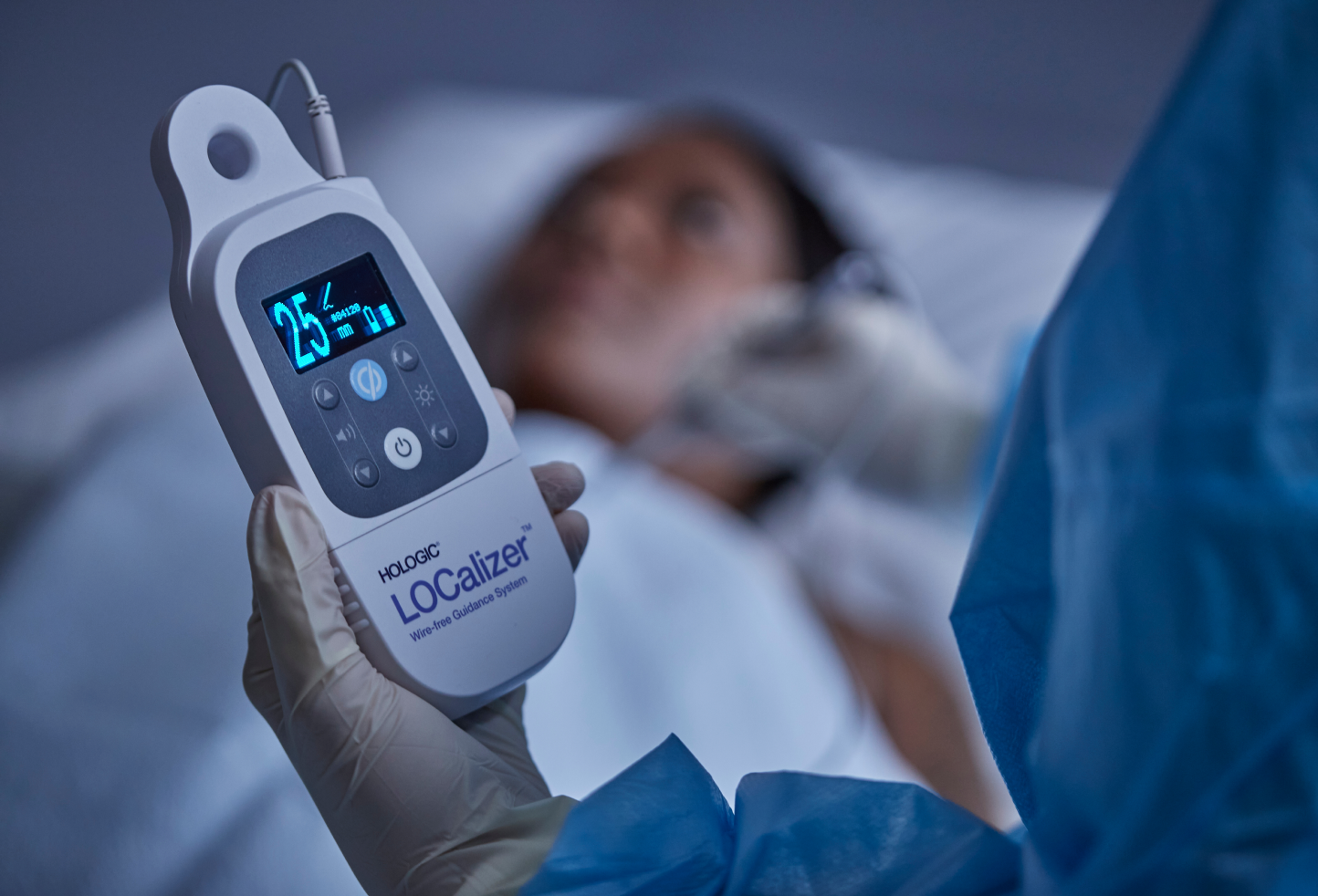
Portable handheld reader that can be placed in the sterile field (when bagged in sterile drape (included in the system).
At approximately 11mm long and 2mm in diameter, each Tag includes a unique identification number displayed by the reader and a polypropylene cap designed to prevent migration in tissue. The Tag is visible under X-ray and ultrasound and has no contraindication for patients with nickel allergies.
The Surgical Probe is a pencil-sized, single-use sterile probe that will guide the surgeon towards the Tag during the operation. The 8mm diameter of the probe allows for small incisions.
The handheld Reader displays the distance to the Tag in millimeters and the Tag’s ID number on a bright screen, making it easy to read during the procedure. The LOCalizer system can read the tag at up to a 60mm distance and is designed to function without interference from the other equipment in the OR.