Aptima® HBV Quant Assay
Deliver trusted results with dual targets across a wide linear range and all major HBV genotypes.

Overview
Documents
Training
HBV Treatment Management in Sharp Focus
The Aptima® HBV Quant Assay is a molecular HBV treatment management assay with dual-target design. It helps providers guide treatment management for patients with chronic hepatitis B infection who are undergoing antiviral therapy.
Quantitation Across Genotypes
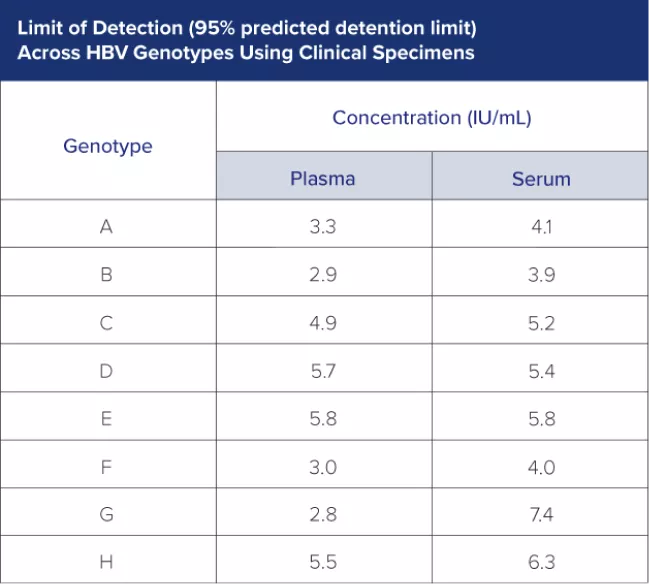
Validated performance across HBV genotypes A – H, with added tolerance to mutations.
Unique Design
Allows laboratorians to deliver trusted results from baseline throughout treatment despite low or high viral loads.
Quantitate with Confidence
The Aptima HBV Quant assay utilizes a unique dual-target design, providing accuracy in the face of high and low viral loads, genotype variation and mutations, so you can deliver trusted results from baseline measurement throughout treatment.
Unique design. Ultimate confidence.
The Aptima HBV Quant assay is a dual-target molecular assay for HBV treatment management.1
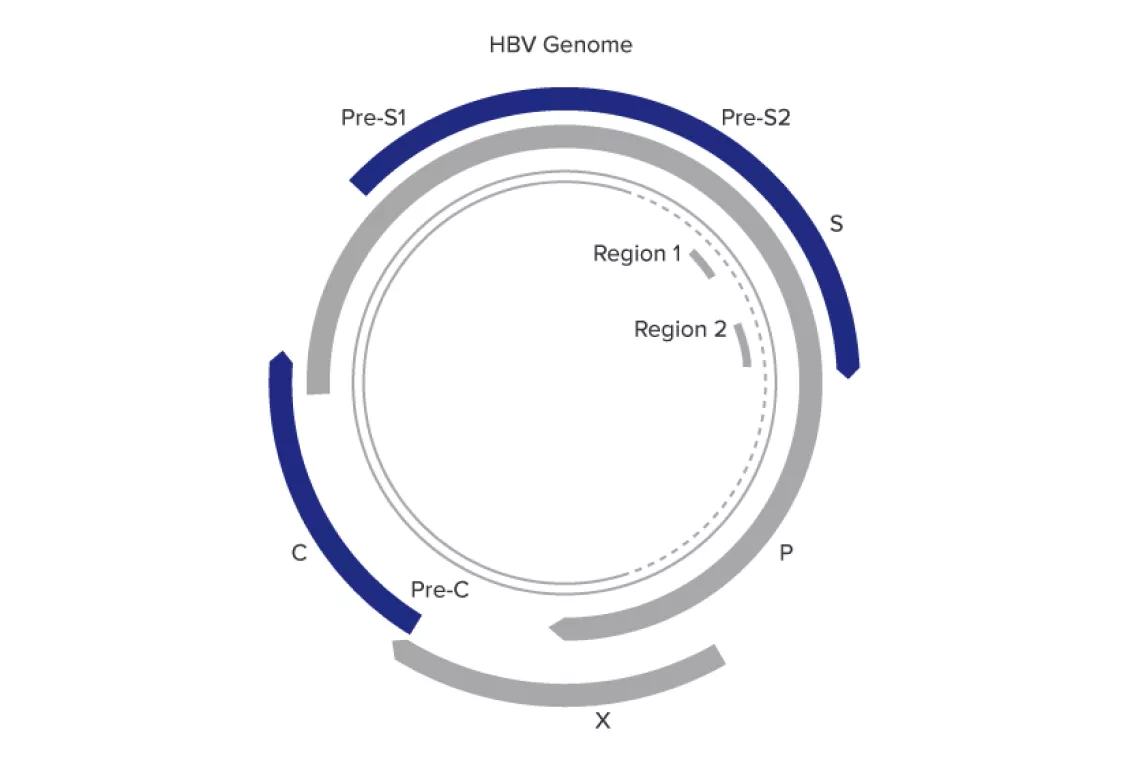
- Targets 2 highly conserved regions in the polymerase and surface genes
- Provides added tolerance to mutations in the HBV genome
- Ensures accurate quantitation over a wide linear range
Track even low-level concentrations of HBV virus across all major HBV genotypes.
The Limit of Detection (LoD) of the Aptima HBV Quant assay is 4.8 IU/mL in plasma samples and 5.9 IU/mL in serum samples, from only 500 μL of reaction volume.*
1) Limit of Detection (LOD) across HBV genotyping using clinical specimens.
2) The LLoQ of the Aptima HBV Quant assay has been thoroughly and rigorously validated across HBV genotypes A-H with thousands of data points, across multiple reagent lots, instruments and operators.1
*Calculated using the WHO 3rd International Standard
Claim
Quant
LoD
4.8 IU/mL in plasma
5.9 IU/mL in serum
LLoQ
6 IU/mL in plasma
8 IU/mL in serum
Methodology
RT-TMA
Verified Sample Types*
Serum (SST, serum tubes)
Plasma (EDTA, ACD, PPT)
Your Virology IQ
Test Your Virology Knowledge
Take a quiz on current virology statistics and historic innovations.
Question 1 of 2
What are the CDC recommendations for HBV Routine Testing?
- ×
- ×
ANSWER B: Persons born in regions of high and intermediate HBV endemicity (HBsAg prevalence 2%) test for HBsAg, regardless of vaccination status in their country of origin, including immigrants, refugees, asylum seekers, and internationally adopted children. If HBsAg-positive, refer for medical management. If negative, assess for on-going risk for hepatitis B and vaccinate if indicated.
Reference: Adapted from: Centers for Disease Control and Prevention. Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. MMWR 2008; 57 (No. RR-8).
Question 2 of 2
Should pregnant women get tested for HBV?
- ×
- ×
ANSWER A: The CDC recommends that all pregnant women test for HBsAg during each pregnancy, preferably in the first trimester. Test at the time of admission for delivery if prenatal HBsAg test result is not available or if mother was at risk for infection during pregnancy. If HBsAg-positive, refer for medical management. To prevent perinatal transmission, infants of HBsAg-positive mothers and unknown HBsAg status mothers should receive vaccination and postexposure immunoprophylaxis in accordance with recommendations and within 12 hours of delivery.
Reference: Adapted from: Centers for Disease Control and Prevention. Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. MMWR 2008; 57 (No. RR-8).
Results
You’re well on your way! Continue reading about the Virology assay suite.
You’ll feel more informed and empowered when speaking with your healthcare provider.
You’re well on your way! Continue reading about the Virology assay suite. You’ll feel more informed and empowered when speaking with your healthcare provider.
Disclaimer:
* TMA technology is patented and owned by Hologic. In 2014, Grifols, S.A. was granted rights of commercialization of the blood and plasma screening brand, Procleix, a triplex TMA assay. In 2014, Grifols acquired certain assets from Novartis, which, among other things, included the rights to market transfusion medicine assays, Tigris and Panther instruments using TMA technology. The transaction enabled Grifols to offer screening solutions to blood and plasma donation centers. Grifols acquired Hologic’s rights to the blood screening business in 2017.
† CE Marked only. Not for sale in the United States.
References:
1. Gill, P. and Ghaemi, A. 2008. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids. 27(3):224-43. 2. Hill, C. 2001. Molecular diagnostic testing for infectious diseases using TMA technology. Expert Reve. Mol. Diagn. 1(4): 445-455. 3. Data on File. Hologic, Inc. 4. National Science & Technology Medals Foundation. Gen-Probe Incorporated: 2004 National Medal of Technology and Innovation: Medicine. Accessed April 12, 2022. 5. Aptima HIV-1 RNA Qualitative Assay. US package insert. 501623. Hologic, Inc.; 2015. 6. Aptima HCV RNA Qualitative Assay. US package insert 500237. Hologic, Inc.; 2016. 7. Aptima HIV-1 Quant Assay. US package insert AW-13242-001. Hologic, Inc.; 2016. 8. Aptima HCV Quant Dx Assay. US package insert AW-14498-001. Hologic, Inc.; 2018. 9. Aptima HBV Quant assay. US package insert AW-15644_002. Hologic Inc.; 10. Aptima HIV-1 Quant Dx Assay. US package insert AW-18107-001. Hologic, Inc.;
Explore the data
Learn More About the Aptima® Virology assays on the Panther® System.
J Clin Microbiol. 2017 Apr;55(4):1211-1219
J Appl Lab Med. 2019 Nov;4(3):383-390
Clin Chem Lab Med. 2018 Mar 28;56(4):634-641