EcoSac Tissue Retrieval System
Work efficiently in small spaces and utilize smaller ports with the EcoSac tissue retrieval system.

Overview
Documents
Training
Comprehensive Laparoscopic Solutions
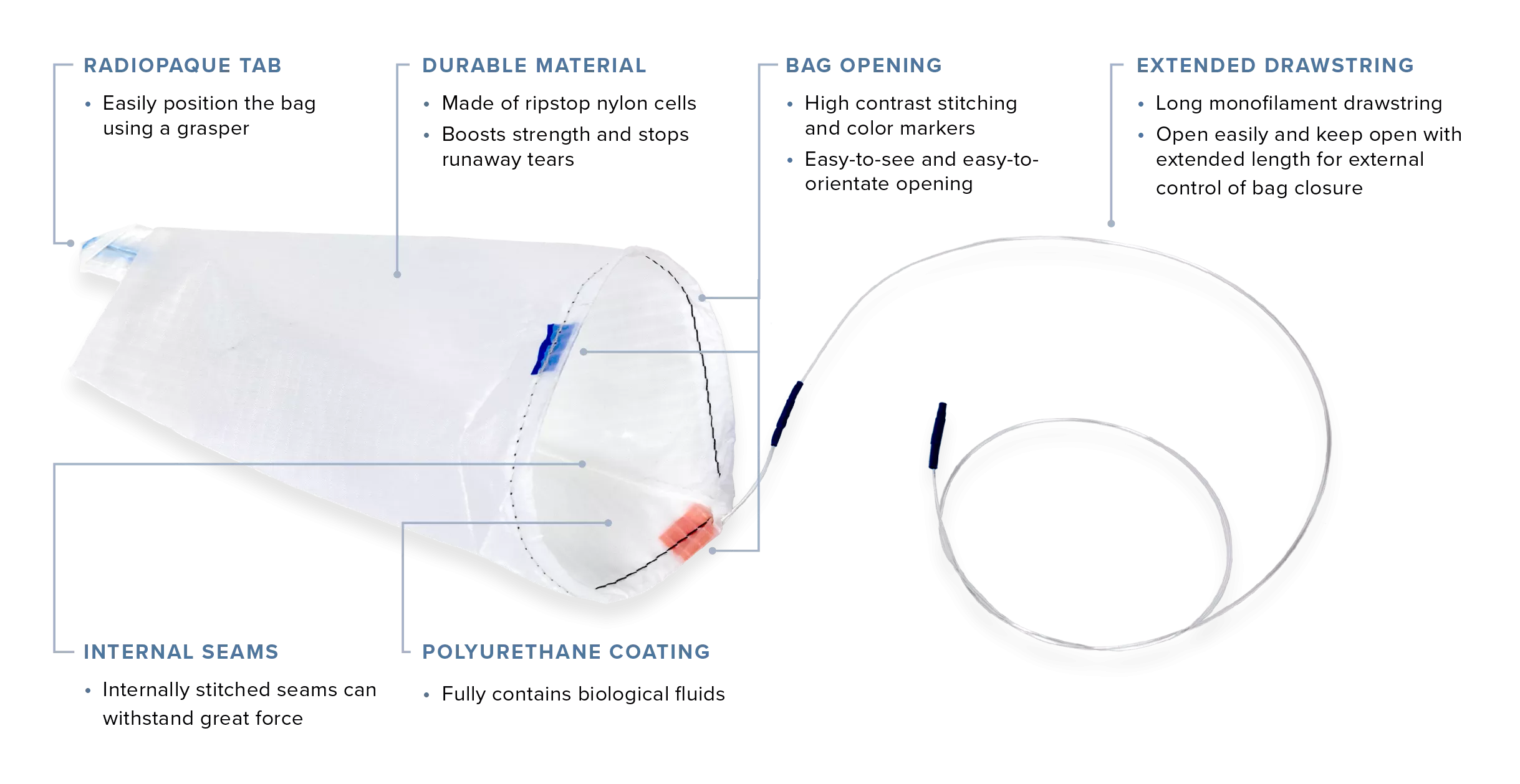
The EcoSac tissue retrieval system feature a unique design that eliminates the need for a plastic introducer and applicator, reducing shelf space requirements and eliminating plastic waste.
User Friendly
Seamlessly reopen and reuse throughout a procedure, with a smooth drawstring closure and nylon that becomes transparent when wet for easy viewing of the specimen.
Eco-and-Environmentally Friendly
Reduce packaging, storage space, waste, and costs without the need for a plastic introducer and applicator.
Strength and Durability
Our unique coated ripstop nylon is resistant to tearing if punctured.
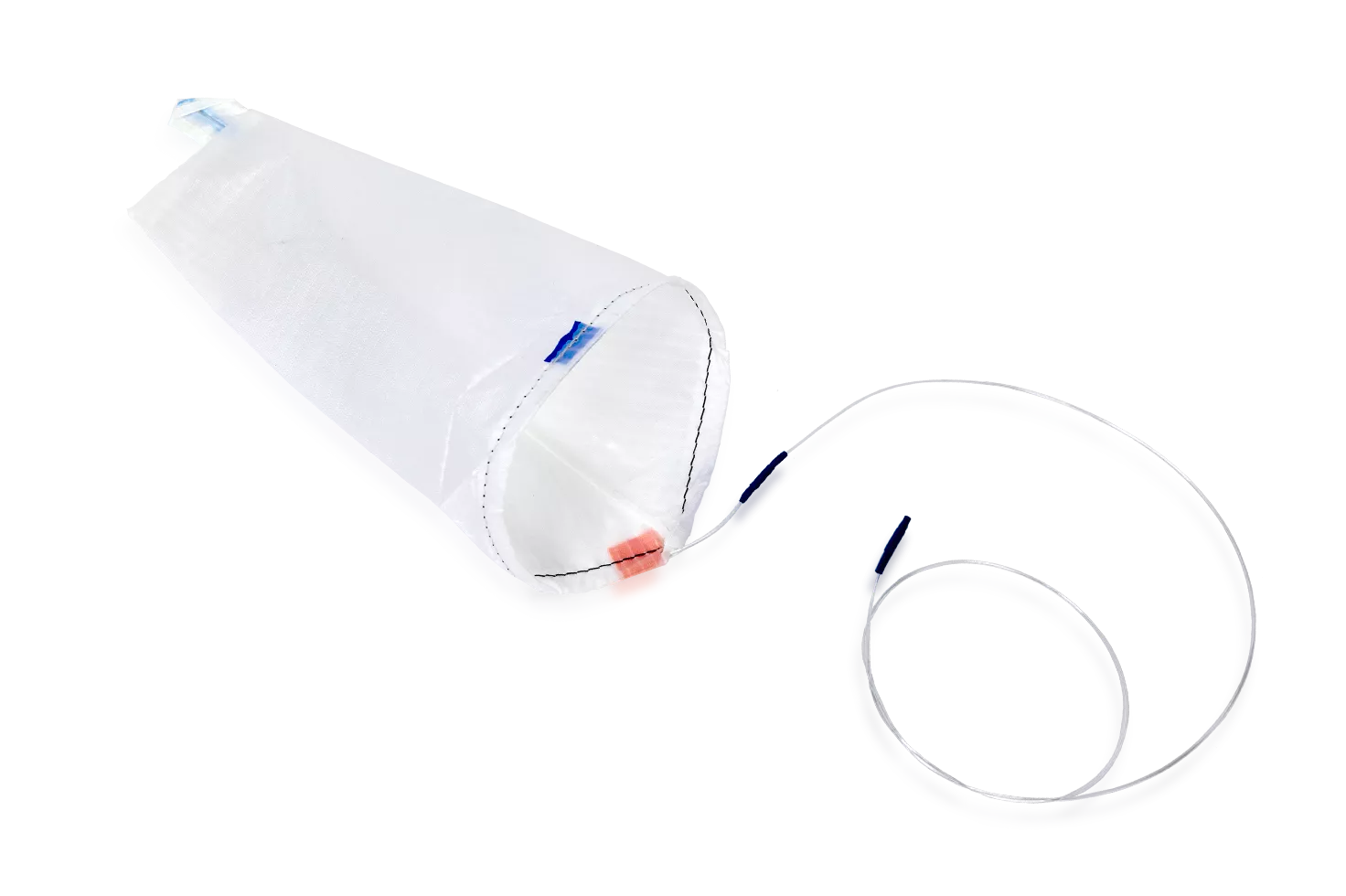
EcoSac Tissue Retrieval System
- Fully deploys during laparoscopic surgery
- EcoSacs have a uniquely coated ripstop nylon that is resistant to tearing
- Options include EcoSac bags that fit down 5mm, 10mm, 12mm, and 15mm trocars.
Product Specifications
Specialties
General, Colorectal, Pediatrics, Urology, GYN, Bariatrics
Procedures
Appendectomy, Lap Cholecystectomy, Nephrectomy, Small bowel resection
Ordering Information
Vizient Award
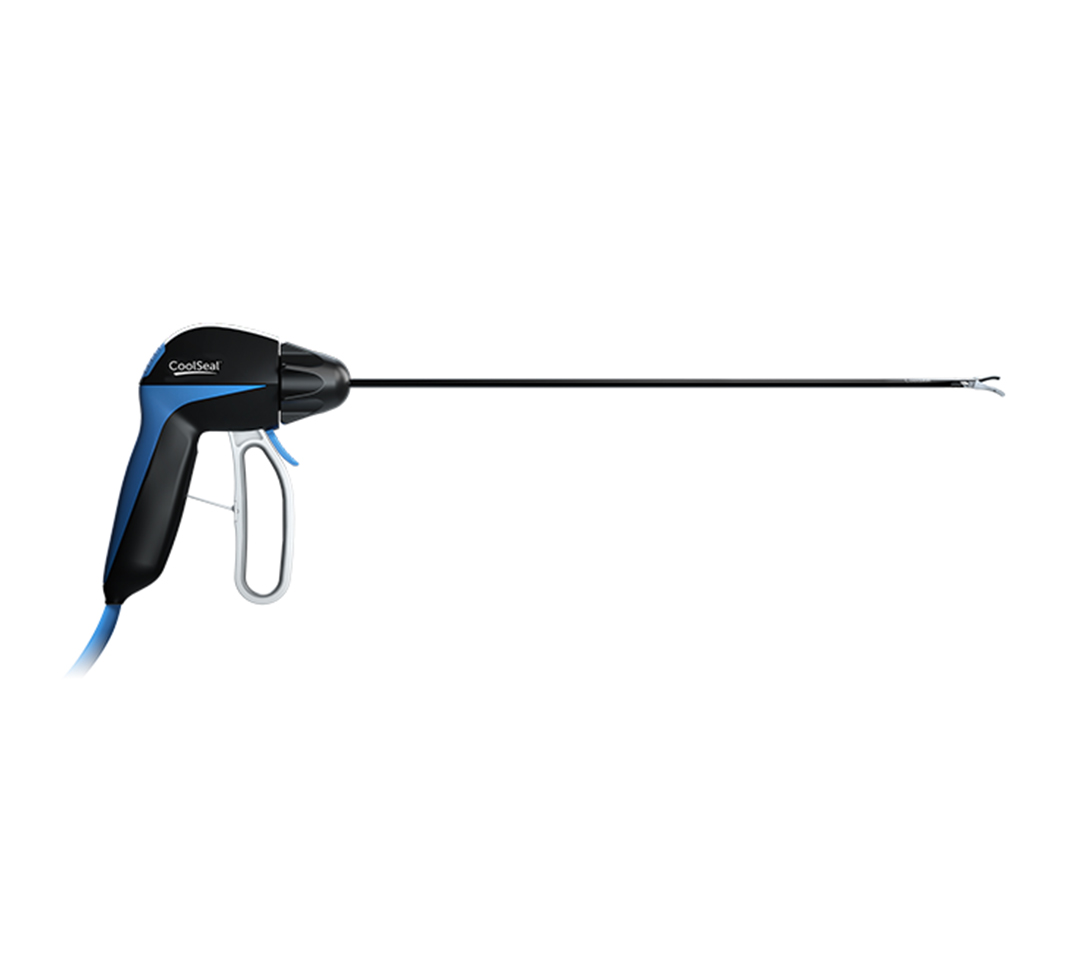
The CoolSeal® advanced energy portfolio and JustRight® 5mm stapler were awarded the Innovative Technology designation from Vizient, Inc., the nation’s largest provider-driven healthcare performance improvement company. Vizient awarded the contract after hospital experts who served on one of its provider-led councils recommended CoolSeal and JustRight.
These provider-led councils evaluate products submitted through Vizient’s Innovative Technology Program. They recommend contracts for technologies that have the potential to enhance clinical care, patient safety and healthcare worker safety, or to improve business operations of healthcare organizations.
View the Hologic press release.
Related Products
Safety Data Sheets
Package Inserts

Overview
Package Inserts
Resources
Syndromic Respiratory Testing.
Patient-Specific Results.
The Panther Fusion Respiratory assays are the premier set of assays on the Panther Fusion® system. You can provide truly personalized syndromic respiratory testing with qualitative detection and differentiation of the most common respiratory viruses from a single patient sample. Each Panther Fusion Respiratory assay can be processed independently or simultaneously with other Panther Fusion® and Aptima® assays.
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV, Panther Fusion® Flu A/B/RSV, Panther Fusion® Paraflu, Panther Fusion® AdV/hMPV/RV and Panther Fusion® Bordetella assays comprise the CE-IVD respiratory testing menu on the fully automated Panther Fusion system. Each is a multiplex, real-time PCR in vitro diagnostic test. These assays can be run on nasopharyngeal (NP) swab specimens obtained from individuals exhibiting signs and symptoms of a respiratory tract infection.1-5 Additionally, the Panther Fusion® SARS-CoV-2 assay has received Emergency Use Authorization for the detection of SARS-CoV-2 from nasopharyngeal, nasal, mid-turbinate and oropharyngeal swab specimens, nasopharyngeal wash/aspirate or nasal wash, and lower respiratory tract specimens.6
Personalized Respiratory Testing
The Panther Fusion assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay§ - Qualitative detection and differentiation of SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion® Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.2
- The Panther Fusion® AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.3
- The Panther Fusion® Paraflu assay - Qualitative detection and differentiation of parainfluenza 1 virus, parainfluenza 2 virus, parainfluenza 3 virus and parainfluenza 4 virus.4
- The Panther Fusion® Bordetella assay† - Qualitative detection and differentiation of Bordetella pertussis and Bordetella parapertussis.5
- The Hologic® SARS-CoV-2 assays*‡ - Qualitative detection of SARS-CoV-2 virus.6-8
Flex Your Ability
With the Panther Fusion Respiratory assays, a single patient specimen can be tested for SARS-CoV-2 as well as other common respiratory viruses which present with overlapping symptoms, boosting efficiency and increasing clinical insight.
Additionally, when you leverage the power of Panther Fusion, your lab can:
- Run more efficiently with full automation from sample-to-result.
- Receive easy-to-interpret results.
- Personalize syndromic respiratory testing by processing multiple assays from a single specimen.
- Control costs by running only the required assays and reduce expense of unnecessary tests.
- Eliminate the need for reagent preparation with Panther Fusion’s ready-to-use format.
- Reduce waste with 60 day on-board reagent stability.
- Consolidate menu onto a single, fully automated platform with the ability to run both Aptima and Panther Fusion assays alongside each other at the same time.
Resources
Education