Aptima® Multitest Swab Specimen Collection Kit
Consolidate sexual and vaginal health testing onto a single collection device.

Overview
Documents
Training
Package Inserts
Women’s health testing streamlined with just one swab.
With innovative nucleic acid amplification test (NAAT) technology and a single collection device, the Aptima Multitest Swab simplifies testing and provides more answers for sexual and vaginal health.
Accurate Detection and Diagnosis
The Aptima Multitest Swab can be used to collect samples from a variety of specimen collection sites to provide accurate diagnosis for patients.
CDC’s Recommended Collection Method
The vaginal swab is the preferred collection method for chlamydia and gonorrhea1
Identify More Infections
Urine samples can miss up to 10% of underlying infections1
Vaginal samples can miss fewer than 1% of infections*2
Improve Patient Care
Decrease patient callbacks for re-collection when reflex or add-on testing is ordered
One Step Workflow
The Aptima Multitest Swab with the penetrable cap offers laboratories the benefit of an automated workflow developed with laboratory professionals in mind.
- Eliminate manual uncapping, recapping and specimen transfer
- Reduce human error and repetitive motion injures
- Protect against cross-contamination
- Feel reassured with guanidine free, non-toxic media
Enhanced Workflow
Streamline laboratory workflows with a collection device that directly loads on the Panther® system.

Proven Performance
Our portfolio offers a broad assay menu that provides timely and accurate results to empower patients to protect their sexual and reproductive health.
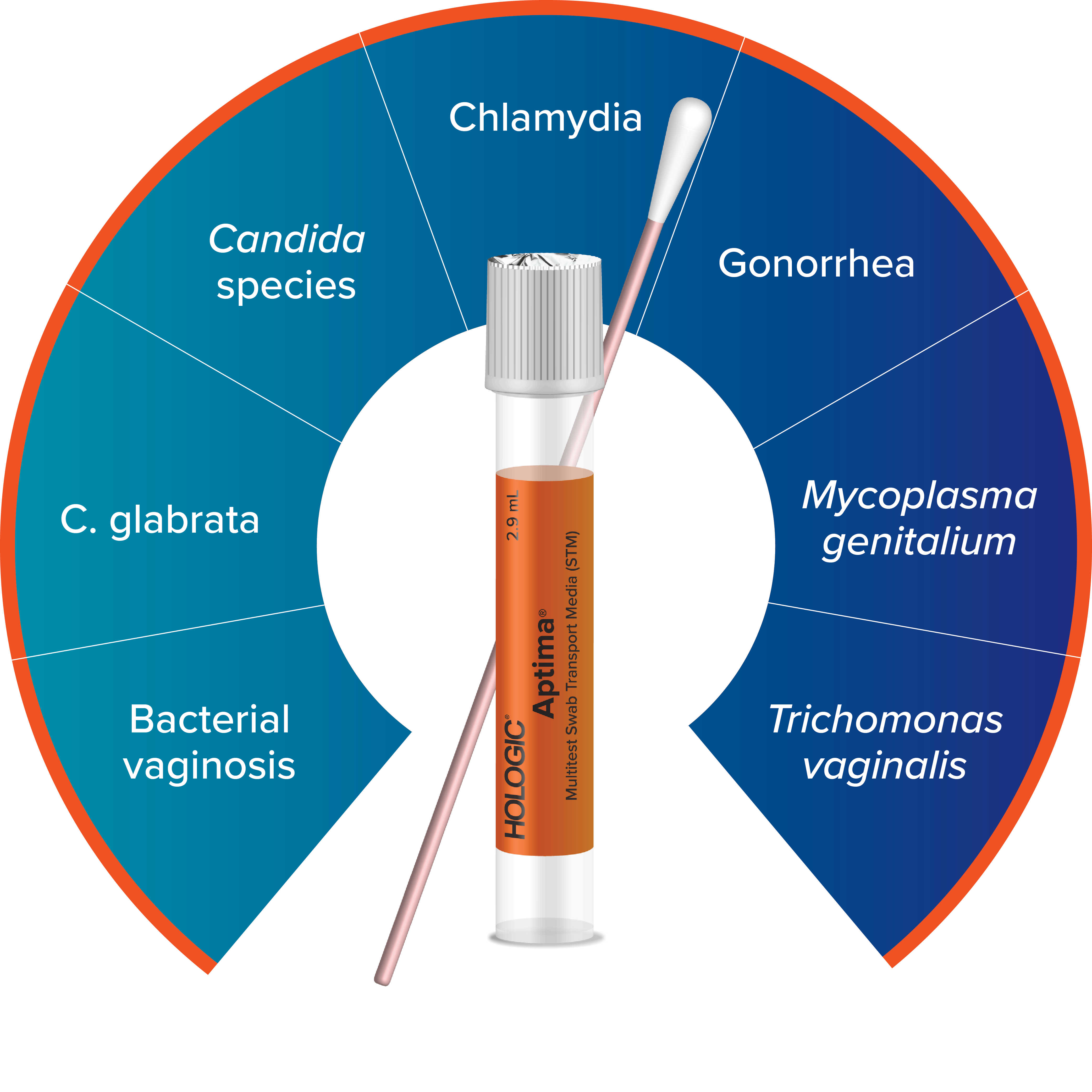
One Sample, Multiple Results
Detect up to 7 infections and disease states with just one vaginal swab.2-6
Aptima® Sexual & Vaginal Health Solution
Learn how our tests help patients protect their sexual and reproductive health.