Overview
Documents
Training
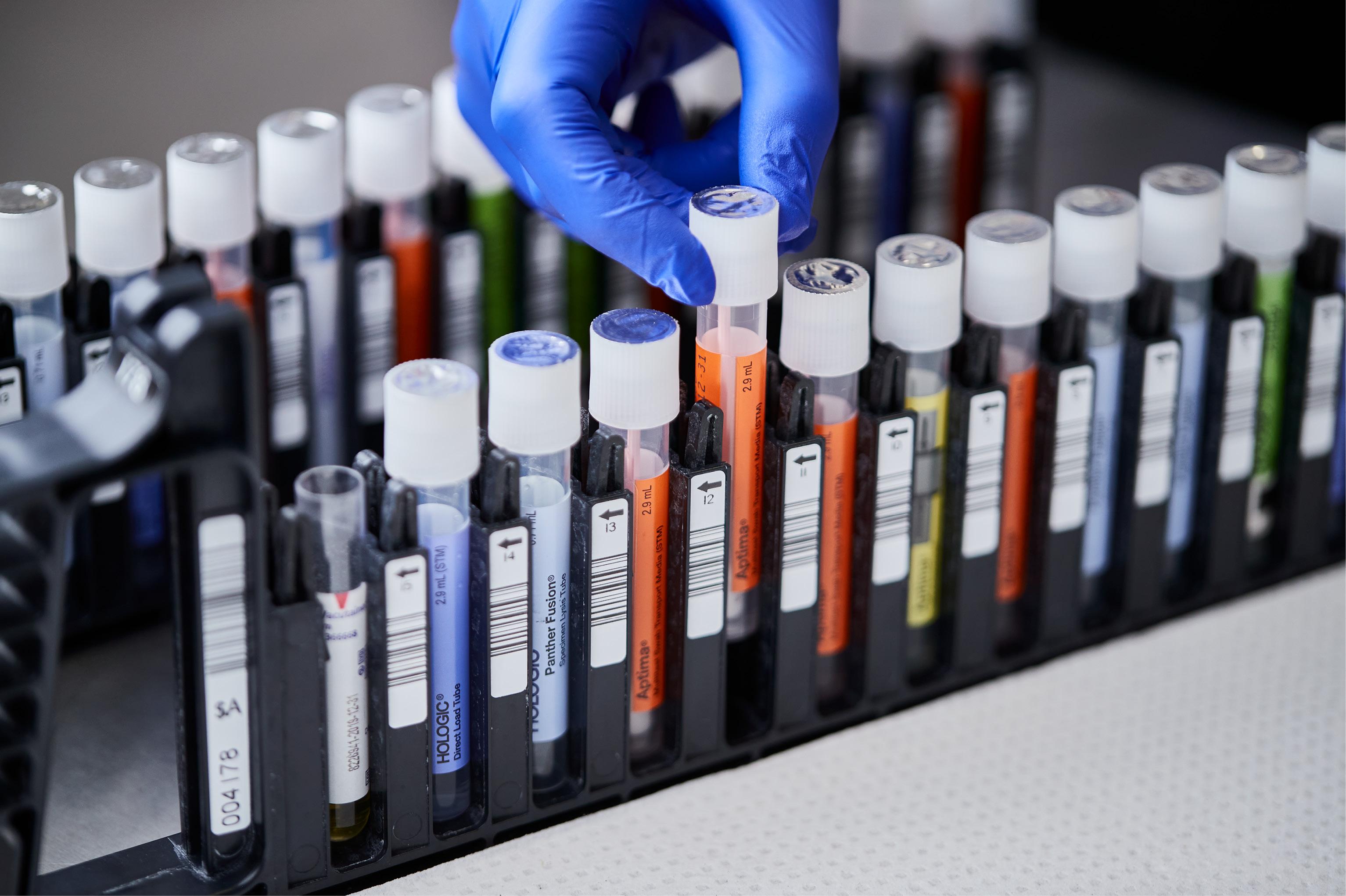
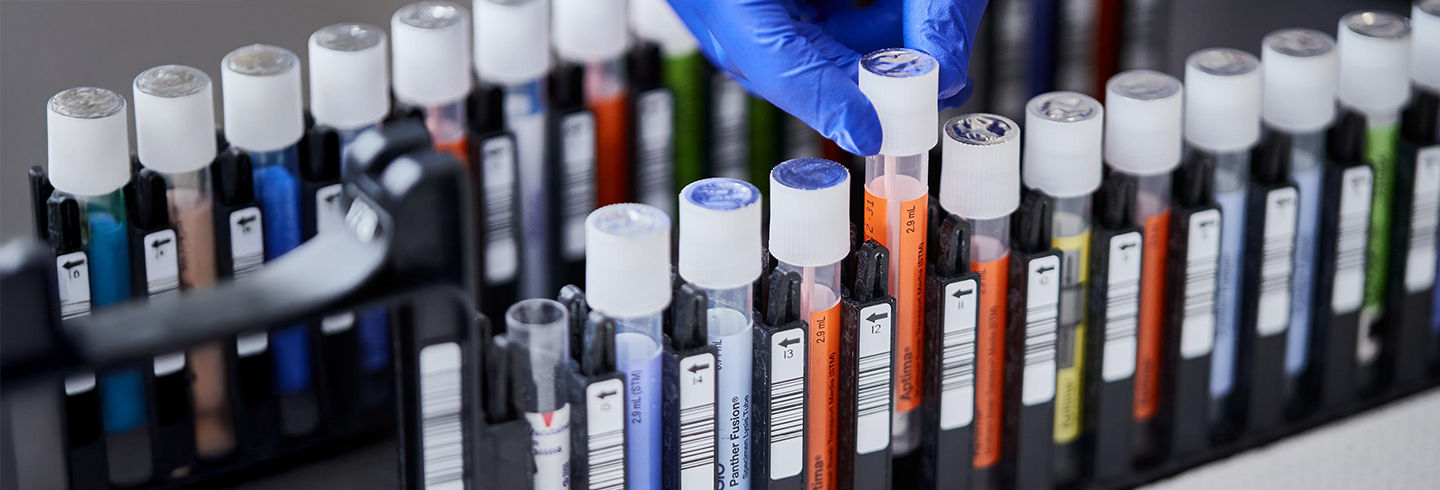
Consolidate testing with direct load collection devices.
With a simplified workflow, market-leading diagnostic tests and scalable automation, you can trust Hologic as your long term testing partner.
One-Step Workflow, Deliver Results Sooner
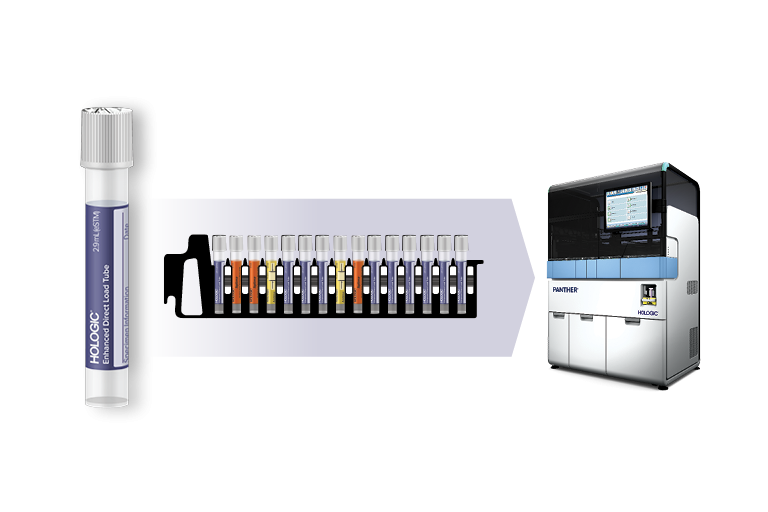
Load directly into the Panther® System
- Directly load alongside specimen types for other assays with true random and continuous access.
- Non-hazardous lysis buffer reduces risk by inactivating common respiratory viruses, minimizing biosafety needs.
- Penetrable cap creates additional cross contamination barrier and eliminates uncapping and recapping of tube.
Market Leading Diagnostic Tests
Hologic's collection devices support a comprehensive testing portfolio for women's health and infectious disease that has been rigorously developed for highly accurate and reliable results.

Scalable Automation
Panther® Scalable Solutions allows you to expand your testing menu while increasing flexibility, capacity and walkaway time. Load samples with Hologic’s collection devices or transfer tubes at any time with true random and continuous access, expediting turnaround time.
Collection Devices

Penetrable Cap
The Aptima Multitest Swab Specimen Collection Kit is intended to be used for collection of the following swab specimen types: vaginal, rectal, throat, penile meatal, nasal and anogenital lesions. Learn More.
Assay Menu
CT/NG
Mycoplasma genitalium
Trichomonas vaginalis
Bacterial vaginosis
Candida vaginitis/Trichomonas vaginalis
HSV 1 & 2

Penetrable Cap
The Aptima Unisex Swab Specimen Collection Kit is used for the collection of female endocervical or male urethral swab specimens. It is also intended for use in sampling other previously collected specimens for processing, extraction, and analysis with other Hologic products.
Assay Menu
CT/NG
Mycoplasma genitalium
Trichomonas vaginalis

Penetrable Cap
The Aptima Urine Specimen Collection Kit is intended to be used for the collection and transport of male or female urine specimens.
Assay Menu
CT/NG
Trichomonas vaginalis
Mycoplasma genitalium
Zika Virus†

Penetrable Cap
The RespDirect™ Collection Kit includes an Enhanced Direct Load Tube (eDLT) and is intended to be used for collection of nasopharyngeal swab specimen. Learn More.
Assay Menu
Panther Fusion SARS-CoV-2/Flu A/B/RSV Assay
Aptima SARS-CoV-2 Assay
Transfer Tubes

Penetrable Cap
The Aptima Specimen Transfer Kit is intended for use with Aptima assays for the testing of gynecological specimens collected in ThinPrep® Pap Test vials containing PreservCyt® solution. It is also intended for use with other Aptima assays and other Hologic products.‡
Assay Menu
CT/NG
Trichomonas vaginalis
HSV 1 & 2
HPV
HPV 16 18/45

Penetrable Cap
Specimen Lysis Tubes and Specimen Transport Medium (STM) are intended to be used for processing specimens for use with Hologic assays.‡
Assay Menu
Flu A/B/RSV
Paraflu
AdV/hMPV/RV
SARS-CoV-2
SARS-CoV-2/Flu A/B*

Aptima Specimen Aliquot Tubes (SAT) are intended for use with plasma and serum specimens that will be tested with Aptima quantitative assays. SATs are included in the Aptima Specimen Diluent Kit.‡