Overview
Documents
Training
Accurate and Customizable Testing for Respiratory Viruses
Since many respiratory infections share common symptoms, it can be difficult to determine which pathogens may be present. Hologic’s menu of Panther Fusion® and Aptima® respiratory assays offer a flexible, mini-panel approach to multiplexed respiratory testing.
With the versatility and automation of the Hologic respiratory portfolio, your lab can process any combination of our respiratory assays from one patient sample. Provide truly personalized syndromic respiratory testing for your lab and patients with qualitative detection and differentiation of the most common respiratory viruses including Flu A, Flu B, RSV, SARS-CoV-2, parainfluenzas 1-4, adenovirus, human metapneumovirus, and rhinovirus. The choice is yours.
Discover the Flexibility of Hologic’s Multiplexed Assays
Expand your respiratory capabilities year-round; process as few as 1 or up to 1,000 samples in 24 hours with scalable automation.1
Personalize Testing
Eliminate unnecessary patient costs and only test for what they need. Test one sample across several disease states with a customizable multiplexed assay menu.
Automate Processing
Do more without extra personnel. Increase your walk-away time with Hologic’s direct load tubes and increase efficiency with random and continuous access on the fully automated Panther® system.
Optimize Operations
Streamline your lab's workflow. Consolidate assays on a system that allows up to 32 assay reagent kits on-board. Panther Fusion's ready-to-use reagent cartridges have 60-day on-board stability.2
Why Hologic’s respiratory assays?
Hear from customers who trust Hologic’s respiratory assays and direct load collection devices during respiratory season and beyond.
"With Panther, I am able to manage unpredictable COVID surges along with the routine testing that we have on a daily basis.”
Customized Syndromic Respiratory Testing
The Panther Fusion and Aptima assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab. Protect patient and population health with tailored respiratory testing solutions.
RespDirect™ Collection Kit8
Streamline laboratory workflow with the Hologic RespDirect Collection Kit. After collection, the tube arrives in the lab and is ready to load onto the Panther/Panther Fusion system without any uncapping or specimen transfer steps. The tube features a penetrable cap offering laboratories the benefit of an automated workflow developed with laboratory professionals in mind.
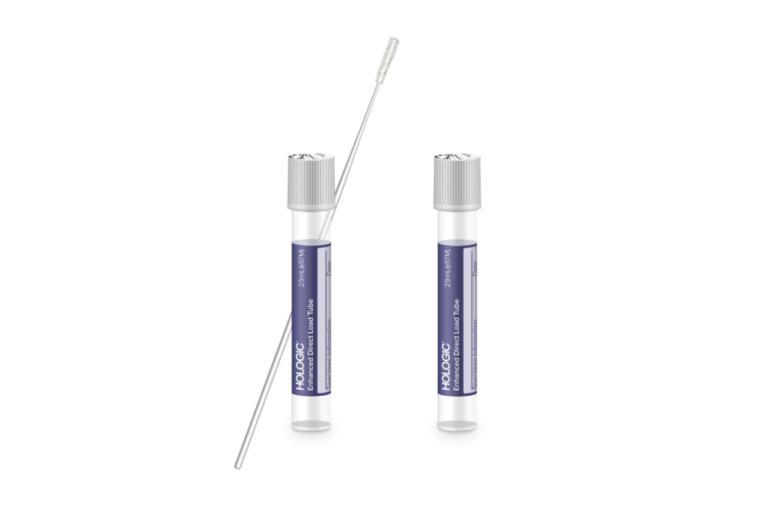
Simplified workflow on a fully automated platform.
Detect and differentiate common respiratory viruses from a single sample with flexible testing algorithms unique to our respiratory portfolio.

![]() Aptima® SARS-CoV-2 Assay
Aptima® SARS-CoV-2 Assay
![]() Panther Fusion® Flu A/B/RSV Assay
Panther Fusion® Flu A/B/RSV Assay
![]() Panther Fusion® AdV/hMPV/RV Assay
Panther Fusion® AdV/hMPV/RV Assay
![]() Panther Fusion® Paraflu Assay
Panther Fusion® Paraflu Assay
![]() Panther Fusion® SARS-CoV-2/Flu A/B/RSV Assay
Panther Fusion® SARS-CoV-2/Flu A/B/RSV Assay
Video Resources

Discover the flexibility of smaller respiratory panels with Panther Fusion and a head-to-head workflow evaluation.
Ordering Information
1. Data on file. 2. Panther/Panther Fusion System Operator’s Manual. AW-26055. 2022. 3. Panther Fusion SARS-CoV-2/Flu A/B/RSV assay. US package insert AW-32326. Hologic, Inc.; 2024. 4. Panther Fusion Flu A/B/RSV assay. US package insert AW-16832. Hologic, Inc.; 2019. 5. Panther Fusion AdV/hMPV/RV assay. US package insert AW-28996. Hologic, Inc.; 2023 6. Panther Fusion Paraflu assay. US package insert AW-16833. Hologic, Inc.; 2019. 7. Aptima SARS-CoV-2 Assay. US package insert AW-32532. San Diego, CA: Hologic, Inc; 2025. 8. Hologic RespDirect Collection Kit. US package insert AW-32530. Hologic, Inc.; 2024.
Documents
Safety Data Sheets
Package Inserts
Related Products

Overview
Package Inserts
Resources
Syndromic Respiratory Testing.
Patient-Specific Results.
The Panther Fusion Respiratory assays are the premier set of assays on the Panther Fusion® system. You can provide truly personalized syndromic respiratory testing with qualitative detection and differentiation of the most common respiratory viruses from a single patient sample. Each Panther Fusion Respiratory assay can be processed independently or simultaneously with other Panther Fusion® and Aptima® assays.
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV, Panther Fusion® Flu A/B/RSV, Panther Fusion® Paraflu, Panther Fusion® AdV/hMPV/RV and Panther Fusion® Bordetella assays comprise the CE-IVD respiratory testing menu on the fully automated Panther Fusion system. Each is a multiplex, real-time PCR in vitro diagnostic test. These assays can be run on nasopharyngeal (NP) swab specimens obtained from individuals exhibiting signs and symptoms of a respiratory tract infection.1-5 Additionally, the Panther Fusion® SARS-CoV-2 assay has received Emergency Use Authorization for the detection of SARS-CoV-2 from nasopharyngeal, nasal, mid-turbinate and oropharyngeal swab specimens, nasopharyngeal wash/aspirate or nasal wash, and lower respiratory tract specimens.6
Personalized Respiratory Testing
The Panther Fusion assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay§ - Qualitative detection and differentiation of SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion® Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.2
- The Panther Fusion® AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.3
- The Panther Fusion® Paraflu assay - Qualitative detection and differentiation of parainfluenza 1 virus, parainfluenza 2 virus, parainfluenza 3 virus and parainfluenza 4 virus.4
- The Panther Fusion® Bordetella assay† - Qualitative detection and differentiation of Bordetella pertussis and Bordetella parapertussis.5
- The Hologic® SARS-CoV-2 assays*‡ - Qualitative detection of SARS-CoV-2 virus.6-8
Flex Your Ability
With the Panther Fusion Respiratory assays, a single patient specimen can be tested for SARS-CoV-2 as well as other common respiratory viruses which present with overlapping symptoms, boosting efficiency and increasing clinical insight.
Additionally, when you leverage the power of Panther Fusion, your lab can:
- Run more efficiently with full automation from sample-to-result.
- Receive easy-to-interpret results.
- Personalize syndromic respiratory testing by processing multiple assays from a single specimen.
- Control costs by running only the required assays and reduce expense of unnecessary tests.
- Eliminate the need for reagent preparation with Panther Fusion’s ready-to-use format.
- Reduce waste with 60 day on-board reagent stability.
- Consolidate menu onto a single, fully automated platform with the ability to run both Aptima and Panther Fusion assays alongside each other at the same time.
Resources
Education