As World Leaders in Virology, We Are Committed to Helping End HIV

As we observe HIV Testing Day in the United States, we at Hologic proudly stand behind our commitment to provide superior assays necessary to reduce the spread of HIV. To meet the national initiative called End HIV Epidemic (EHE), programs such as Treatment as Prevention (TasP) and medications such as antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) need reliable diagnostic and monitoring assays for patients on therapy.

Described as “revolutionary” by world health authorities,2 TasP or U=U (undetectable = untransmittable) is now widely accepted science. Many clinical studies show that individuals on ART who are virally suppressed or undetectable (≤ 200 copies/ml) do not transmit HIV to sex partners.3 The efficacy of ART and the evidence showing that U really does equal U, when combined with reliable viral load (VL) monitoring, provides a rationale for the boldest HIV initiatives to date with a goal to achieve near eradication of HIV infection in the U.S., reducing the rate of new infection by 90% beginning in 2030.1
It is no exaggeration to say that implementation and funding of TasP programs can safeguard whole communities against the spread of HIV—but ART can only be prescribed to people who know their status. Early (acute stage) diagnosis can present unique challenges when patients with high suspicion of infection receive a negative antibody test.4 When this occurs, a Nucleic Acid Amplification Test is the only method to confirm this early stage of infection.4,5 Some studies even demonstrate the utility of moving NAAT earlier in the diagnostic algorithm, as NAAT allows laboratorians to detect HIV RNA earlier in the infection progression than antigen/antibody assays due to the known window period.6,7,8
Furthermore, the launch of PrEP medications in the last decade has the potential to become a game changer. PrEP medications have demonstrated up to 99% effectiveness at preventing infection in seronegative persons at high risk of exposure.9 First approved by the FDA in 2012, PrEP lowers the risk of infection spread among serodiscordant sexual partners, and increasing PrEP coverage for viable candidates is integrated to the EHE initiative in the U.S.10
Community laboratories with in-house capacity for molecular testing are at the center of supporting patients on ART and PrEP as they are the ones best positioned to foster the kind of consultative, targeted care needed by people living with HIV infection or risk of infection. In addition to quarterly HIV testing, clinicians also order routine checks for other Sexually Transmitted Infections (STIs). The Panther® system is ideally suited to running Aptima® Virology assays (HIV, HCV, and HBV, as well as STI screenings) in small and medium local laboratories.
Ending HIV infection demands a global effort that includes providing access to diagnostic and treatment solutions to resource-limited regions where prevalence may be highest. To fulfill this need, the Hologic Global Access Initiative (GAI) lowers the cost of critical testing for accurate diagnoses in regions like the African continent, which bears the highest burden of HIV infection.12 In partnership with the Clinton Health Access Initiative (CHAI) and MedAccess (backed by the UK government), our GAI program was the first all-inclusive pricing model ever offered to address the needs of resource-limited regions. It enables eligible nations to acquire Panther systems, Aptima assays, materials, service, and support, and even freight and logistics to run quality diagnostic tests and viral-load monitoring for the most prevalent viral diseases. With 80 Panthers (and counting) delivered, GAI adoption has improved over 6.5 million lives to date.13
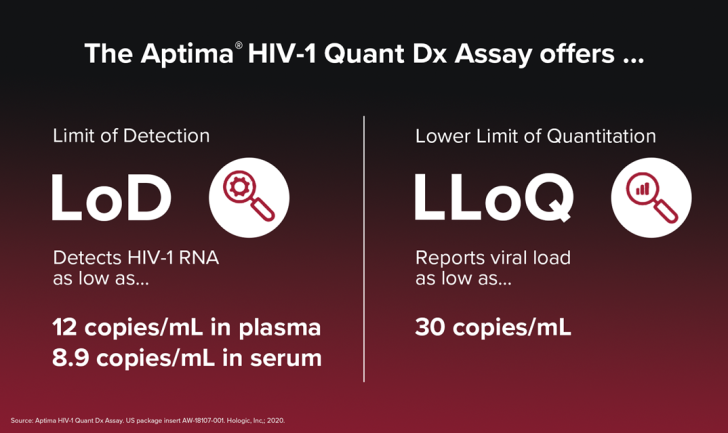
Finally, as the multinational efforts to stop HIV transmission continue, the search for a cure also persists. In that endeavor, we are especially proud that the highly sensitive Aptima HIV-1 Quant Dx has been selected for clinical and scientific studies such as studying HIV reservoirs.14
Hologic is committed to the vision of a world without HIV. Accomplishing this goal after nearly a half-century since its emergence will be one of the great triumphs in modern medicine, combining the efforts of tens of thousands of policy, healthcare, NGO, and laboratory professionals worldwide. As leaders in this endeavor, we have seen firsthand what can be achieved in the fight against HIV, and we echo the call for everyone to get tested and know their status.
Together, we can live with HIV until the day we cure it.