Aptima® HCV Quant Dx Assay
Confirmation of active HCV infection and monitoring HCV viral load on the fully automated Panther® system.
Overview
Documents
Training
Demand More from Your HCV NAAT
The Aptima HCV Quant Dx assay detects and quantitates HCV RNA and is intended for use as an aid in:1
- Diagnosis of active HCV infection following a positive HCV antibody test
- Clinical management of patients infected with HCV
The Aptima HCV Quant Dx Assay intended use is to:
Confirm HCV Infection
Detects and quantitates HCV RNA genotypes 1, 2, 3, 4, 5, and 6.
Monitor Viral Load
Provides industry-leading sensitivity in even the lowest-level HCV concentrations.
HCV Treatment Is Evolving. Now HCV Testing Is Too.
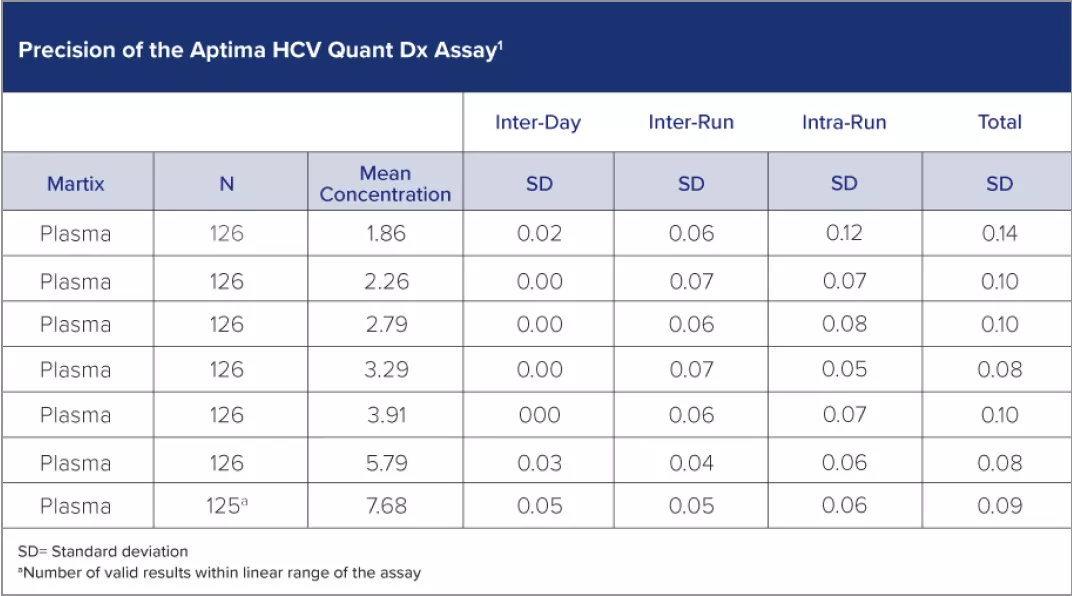
The Aptima HCV Quant Dx assay meets the 2021 American Association for the Study of Liver Diseases (AASLD) recommendations for an FDA-approved quantitative or qualitative nucleic acid test (LoD ≤25 IU/mL) to determine sustained virological response (SVR). It employs real-time transcription-mediated amplification (RT-TMA) technology on Hologic’s Panther® system—and gives you unparalleled performance across several essential parameters.2
Performance by design
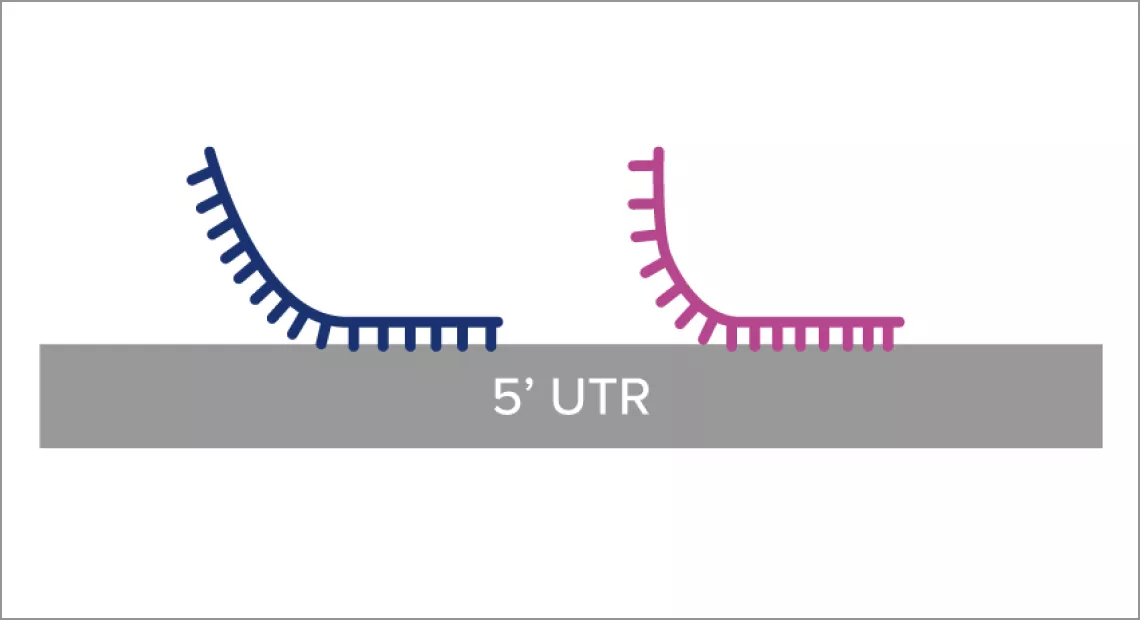
The robust design of the Aptima HCV Quant Dx assay incorporates multiple primers and probes within a highly conserved region of HCV RNA (5’ UTR) to detect and quantitate across genotypes 1 – 6:1
- Redundant target capture oligos for protection against mutations
- Longer oligos for extra protection against single-base mismatches
- Multiple amplification primers (T7 and non-T7) for broad genotype coverage
Redefining sensitivity: Detect even the lowest-level concentrations of HCV across all major genotypes and quantify HCV for optimal treatment monitoring.
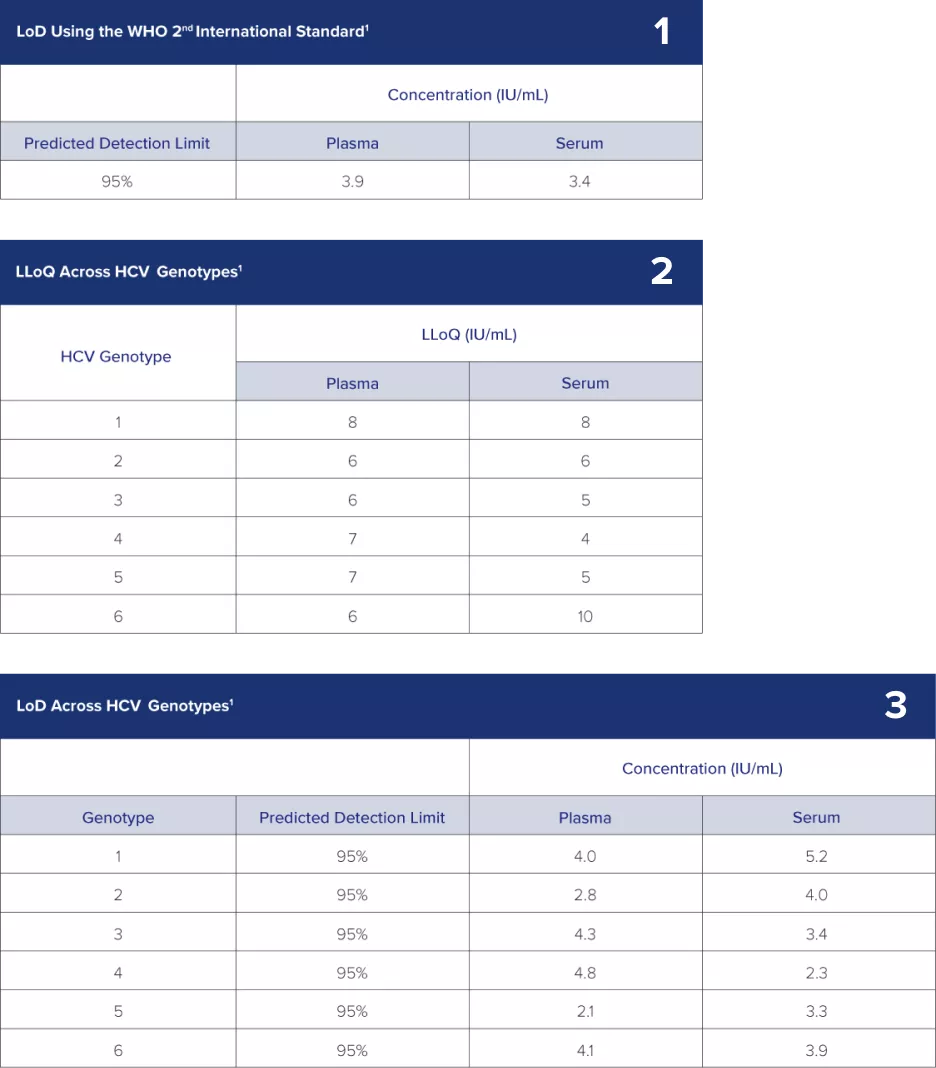
1) Limit of Detection (LoD): The Aptima HCV Quant Dx assay can detect HCV RNA as low as 3.9 IU/mL in plasma samples and 3.4 IU/mL in serum samples.1
2) The LoD has been thoroughly verified across HCV genotypes 1–6 with multiple replicates and multiple reagent lots.1
3) Lower Limit of Quantitation (LLoQ): The Aptima HCV Quant Dx assay LLoQ has been thoroughly established and verified across all major genotypes (1–6).1
Claim
Dx, Quant
LoD
3.9 IU/mL plasma
3.4 IU/mL serum
LLoQ
10 IU/mL
Methodology
RT-TMA
Verified Sample Types*
Serum (SST, serum tubes)
Plasma (EDTA, ACD, PPT)
Your Virology IQ
Test Your Virology Knowledge
Take a quiz on current virology statistics and historic innovations.
Question 1 of 2
Today’s Hepatitis C treatments generally have cure rates of?
- ×
- ×
Question 2 of 2
What are the current CDC recommendations for regular HCV testing?
- ×
- ×
Results
You’re well on your way! Continue reading about the Virology assay suite.
You’ll feel more informed and empowered when speaking with your healthcare provider.
You’re well on your way! Continue reading about the Virology assay suite. You’ll feel more informed and empowered when speaking with your healthcare provider.
Disclaimer:
* For quantitative measurements: Tubes containing EDTA or Acid Citrate Dextrose (ACD) anticoagulants or Plasma Preparation Tubes (PPTs). For qualitative determination: Tubes containing EDTA or ACD anticoagulants, or PPTs, or Serum tubes, or Serum separator tubes (SSTs).
References:
1. Aptima HCV Quant Dx assay. US package insert AW-14498. Hologic, Inc.; 2018. 2. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed April 12, 2022. HIV-1 Quant Dx Assay. US package insert AW-18107-001. Hologic, Inc.;
Explore the data
Learn More About the Aptima® Virology assays on the Panther® System.
J Virol Methods. 2017 Oct;248:159-165
J Clin Virol. 2017 Jul;92:1-6