Fluent® Pro Fluid Management System
Introducing the next generation of fluid management, designed to increase efficiency, confidence and performance in your operating room.

Overview
Documents
Training
Elevate Your Hysteroscopic Procedures.
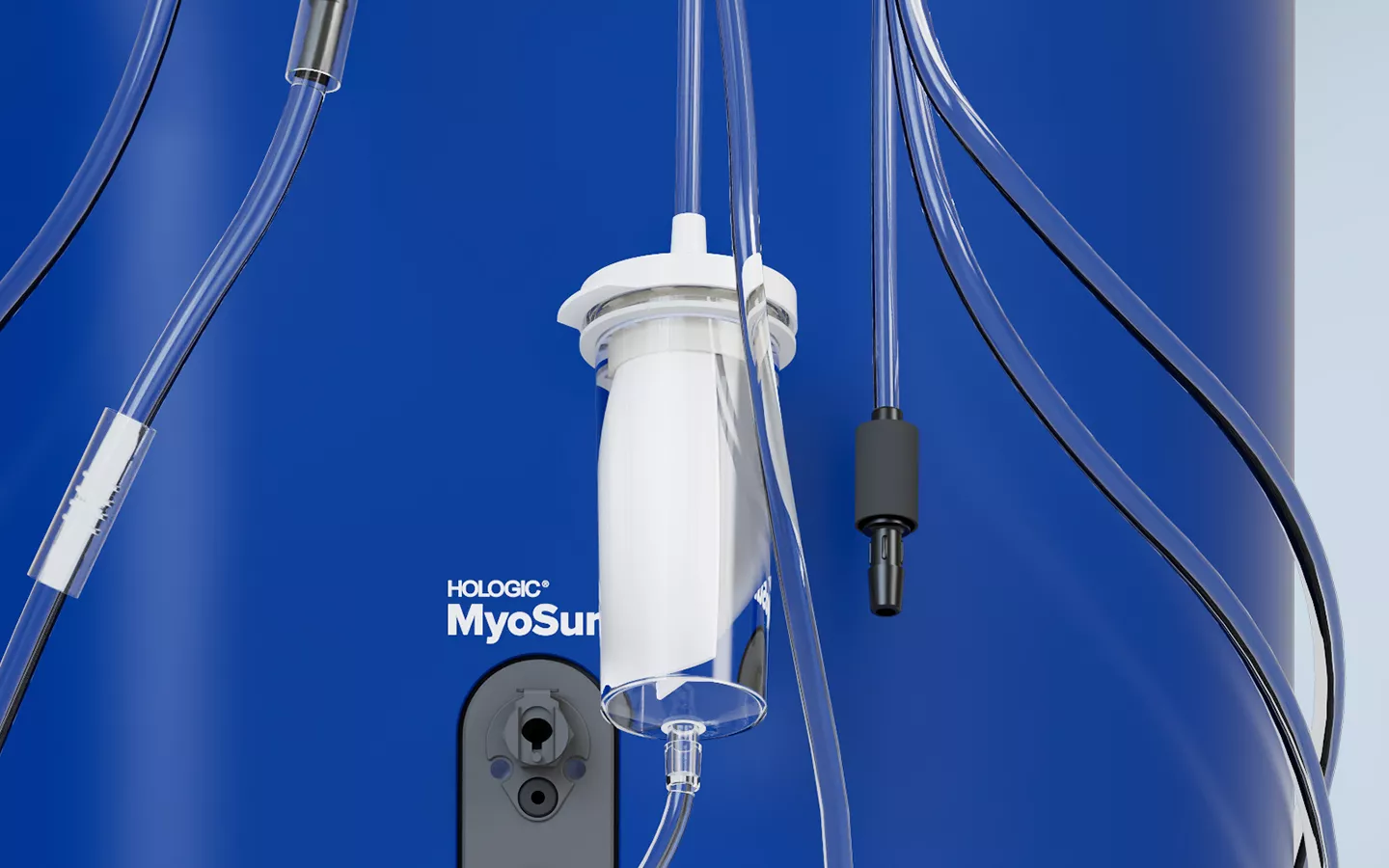
Your hysteroscopic solutions now benefit from our improved fluid management system. The Fluent Pro system was designed to optimize your MyoSure® tissue removal procedures with better suction, visualization and resection.
Achieve up to 4x Faster Suction1
Select between three adjustable levels of suction for resection of simple-to-complex pathology and notice faster draining from under the buttocks drape.1
Experience SmartFlo™ Technology
This unique technology collects inflow rate data and proactively adjusts to deliver consistent pressure during each procedure, optimizing visualization.2
Resect up to 2.5x More Tissue3
Maximize MyoSure tissue resection with the combination of the Fluent Pro system adjustable suction levels and the 10mmHg pressure setpoint decrease during cutting.3
Better Together™
The Fluent Pro fluid management system is designed to optimize your MyoSure tissue removal procedures.
The Fluent Pro system high suction setting resects:1
2.5x more tissue with MyoSure XL device.
2x more tissue with MyoSure Lite device.
1.7x more tissue with MyoSure Reach device.
Experience the power of the MyoSure + Fluent Pro system.
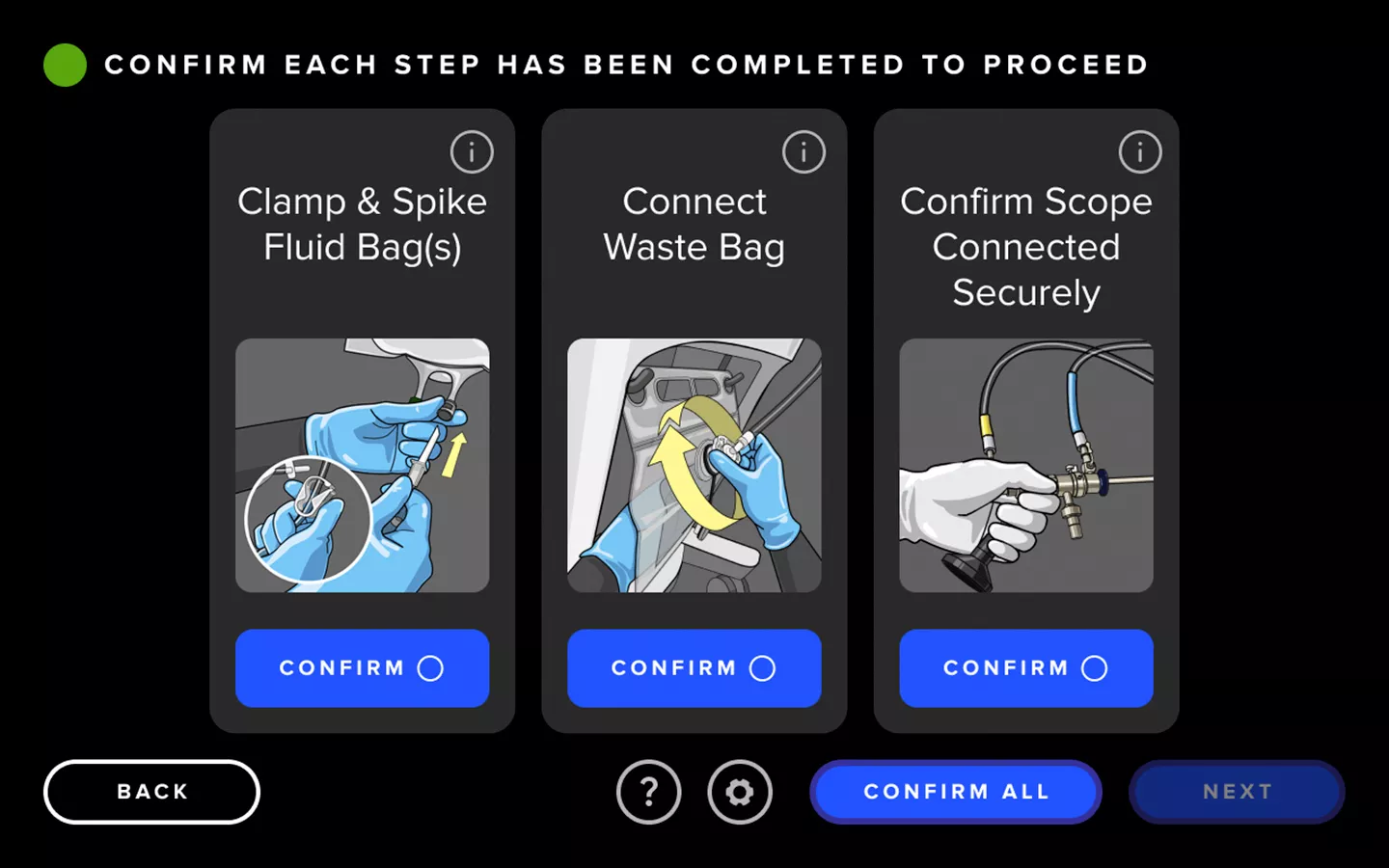
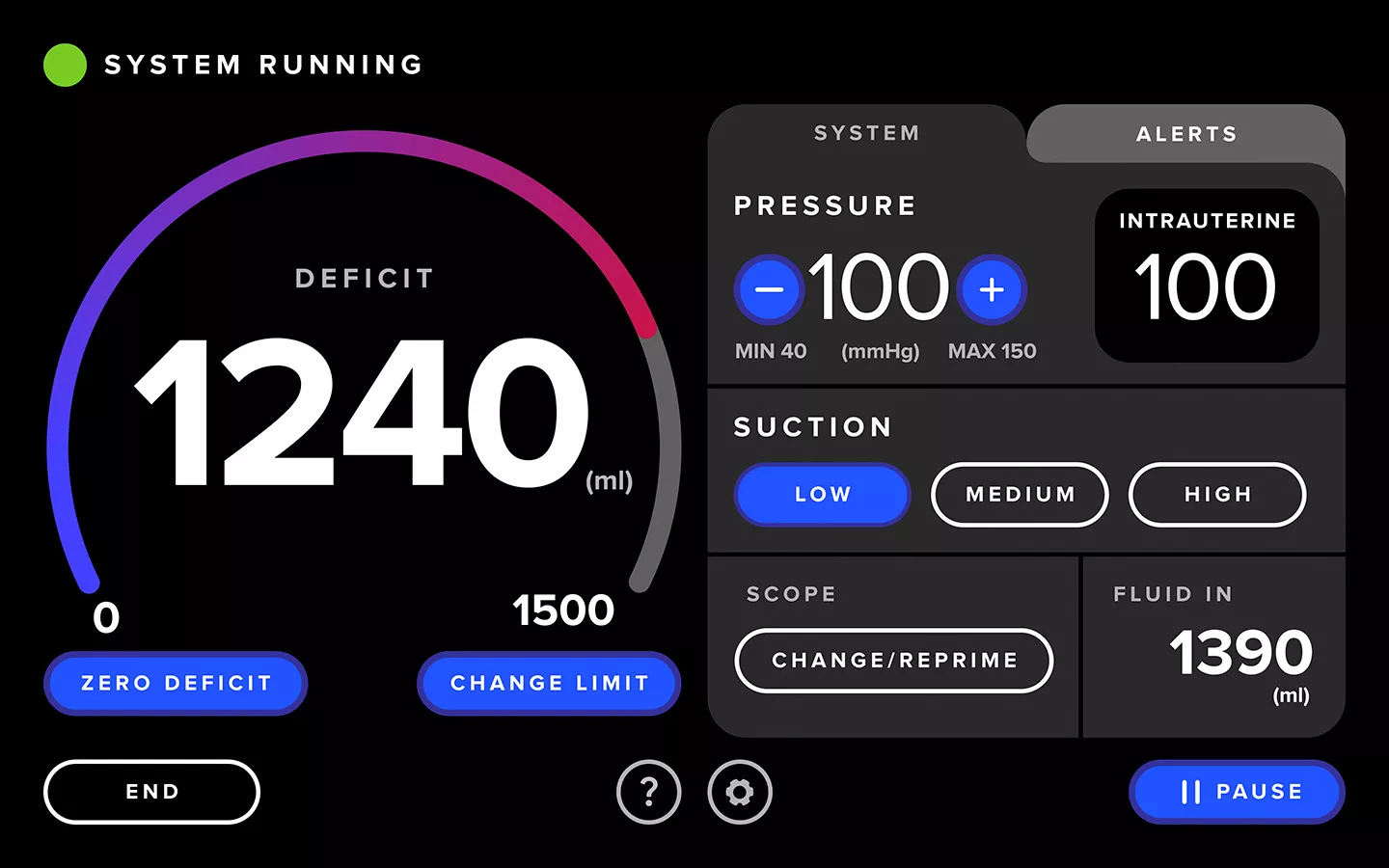
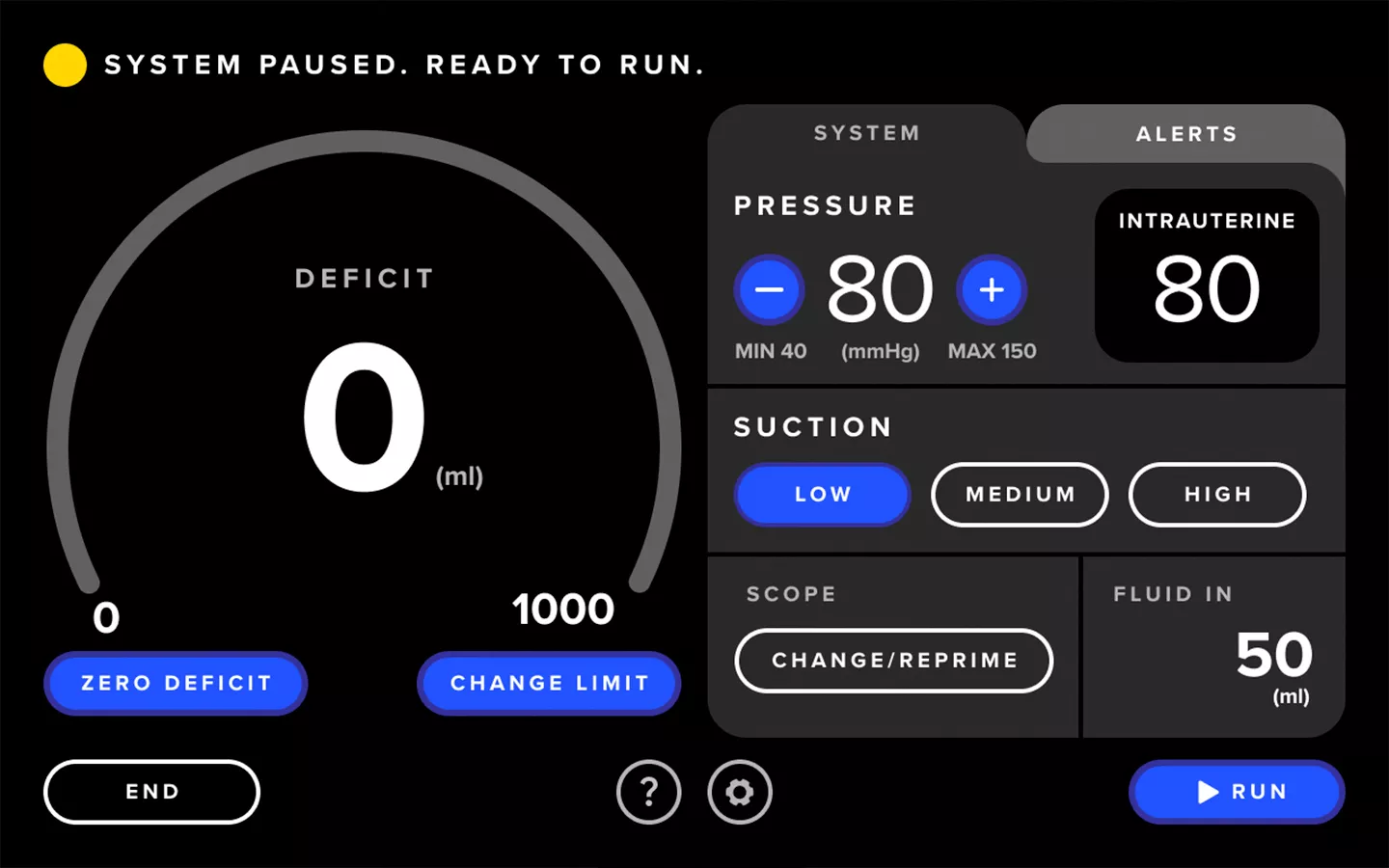
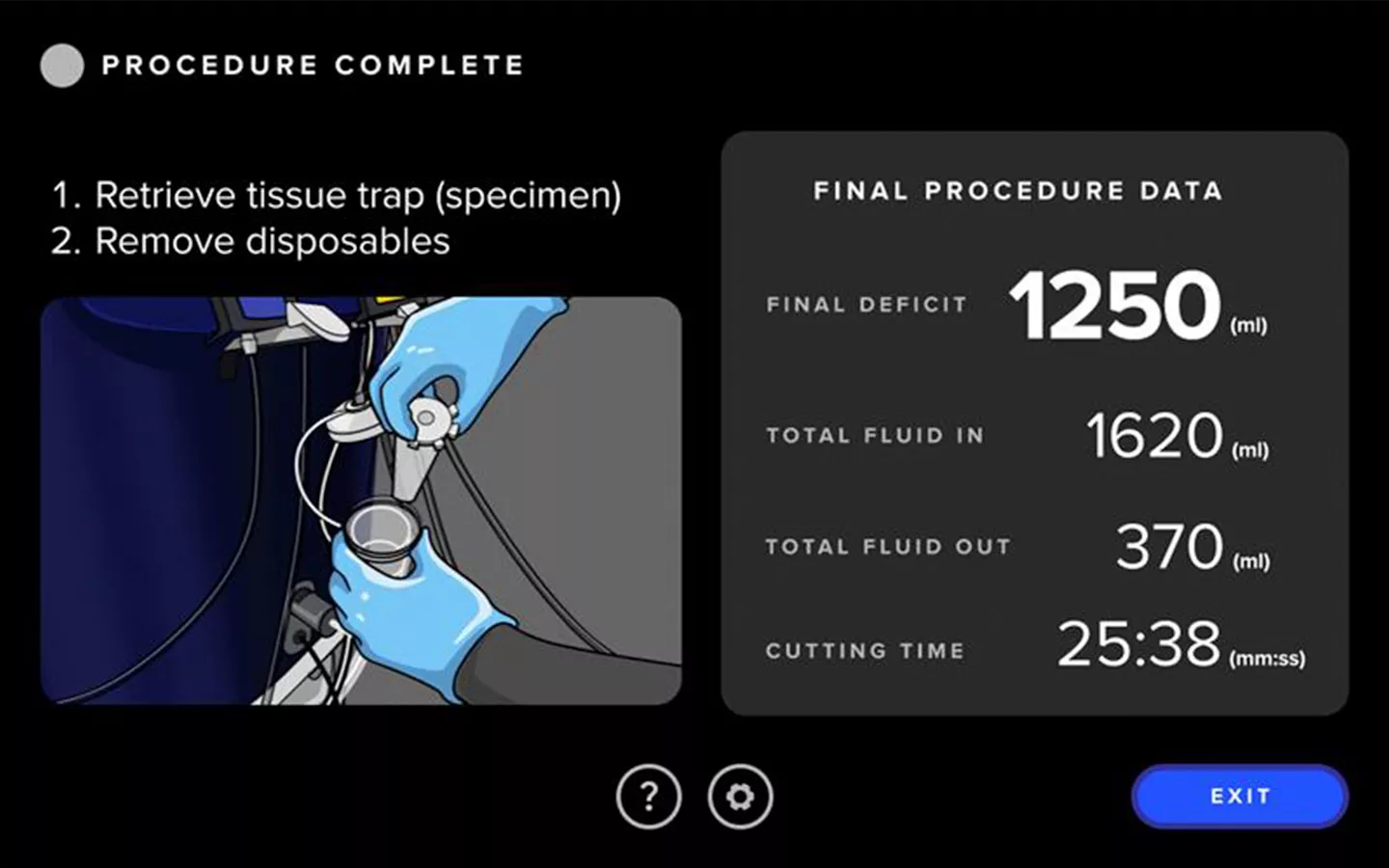
Enhanced User Experience
The Fluent Pro system's touchscreen user interface streamlines tissue removal procedures from start to finish, making setup easier, enabling control during the procedure and providing convenient post-procedure data.
Streamlined Workflow
The Fluent Pro fluid management system has a thoughtfully refined console that enables easy setup and practical procedure workflow, with disposable components that simplify post-procedure processes.
1. Based on a comparison of bench testing performed on the Fluent Pro system high suction setting and the Fluent system default/non-adjustable setting. 2. Based on a survey of 34 physician users during Fluent Pro Limited Market Release, 2024. WEB-02128-001_01 3. Based on a comparison of bench testing performed on the Fluent Pro system high suction setting and the Fluent system default/non-adjustable setting using the MyoSure XL device.
Related Products
Documents
Safety Data Sheets
Package Inserts

Overview
Package Inserts
Resources
Syndromic Respiratory Testing.
Patient-Specific Results.
The Panther Fusion Respiratory assays are the premier set of assays on the Panther Fusion® system. You can provide truly personalized syndromic respiratory testing with qualitative detection and differentiation of the most common respiratory viruses from a single patient sample. Each Panther Fusion Respiratory assay can be processed independently or simultaneously with other Panther Fusion® and Aptima® assays.
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV, Panther Fusion® Flu A/B/RSV, Panther Fusion® Paraflu, Panther Fusion® AdV/hMPV/RV and Panther Fusion® Bordetella assays comprise the CE-IVD respiratory testing menu on the fully automated Panther Fusion system. Each is a multiplex, real-time PCR in vitro diagnostic test. These assays can be run on nasopharyngeal (NP) swab specimens obtained from individuals exhibiting signs and symptoms of a respiratory tract infection.1-5 Additionally, the Panther Fusion® SARS-CoV-2 assay has received Emergency Use Authorization for the detection of SARS-CoV-2 from nasopharyngeal, nasal, mid-turbinate and oropharyngeal swab specimens, nasopharyngeal wash/aspirate or nasal wash, and lower respiratory tract specimens.6
Personalized Respiratory Testing
The Panther Fusion assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay§ - Qualitative detection and differentiation of SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion® Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.2
- The Panther Fusion® AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.3
- The Panther Fusion® Paraflu assay - Qualitative detection and differentiation of parainfluenza 1 virus, parainfluenza 2 virus, parainfluenza 3 virus and parainfluenza 4 virus.4
- The Panther Fusion® Bordetella assay† - Qualitative detection and differentiation of Bordetella pertussis and Bordetella parapertussis.5
- The Hologic® SARS-CoV-2 assays*‡ - Qualitative detection of SARS-CoV-2 virus.6-8
Flex Your Ability
With the Panther Fusion Respiratory assays, a single patient specimen can be tested for SARS-CoV-2 as well as other common respiratory viruses which present with overlapping symptoms, boosting efficiency and increasing clinical insight.
Additionally, when you leverage the power of Panther Fusion, your lab can:
- Run more efficiently with full automation from sample-to-result.
- Receive easy-to-interpret results.
- Personalize syndromic respiratory testing by processing multiple assays from a single specimen.
- Control costs by running only the required assays and reduce expense of unnecessary tests.
- Eliminate the need for reagent preparation with Panther Fusion’s ready-to-use format.
- Reduce waste with 60 day on-board reagent stability.
- Consolidate menu onto a single, fully automated platform with the ability to run both Aptima and Panther Fusion assays alongside each other at the same time.
Resources
Education