Hologic Genius AI® Detection Solution
Hologic’s 3D™ Genius AI Detection solution1 enables you to offer superior mammography screening* through accurate markings, fast triaging tools, and optimized point-of-care results.2,3
Overview
Documents
Training
Giving you the Power to Find Additional Cancers
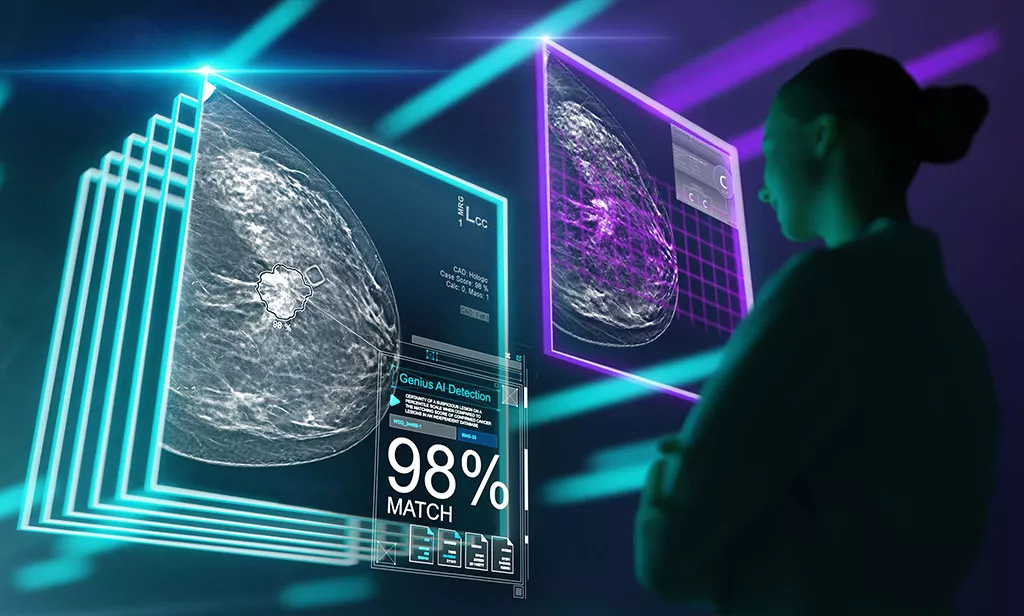
Hologic Genius AI Detection solution is designed to locate lesions likely representing breast cancer with a meticulous search of each slice of the tomosynthesis image set with a high sensitivity of 94%.2,3 Suspicious areas are highlighted at radiologists’ workstations for concurrent reading to support smart, decisive interpretation.
The Future of AI and Breast Cancer Detection
AI Accuracy Sharpens Your Precision
Introducing the new automated lesion correlation feature and over 70% reduction in false positive markings per case compared to Hologic's 2D ImageChecker® CAD.4
Optimize your Workflow Starting with Results in the Exam Room
Immediate access to results right on the acquisition workstation allows for point-of-care triaging and faster response to patients.
Know What to Expect Before Reviewing a Study
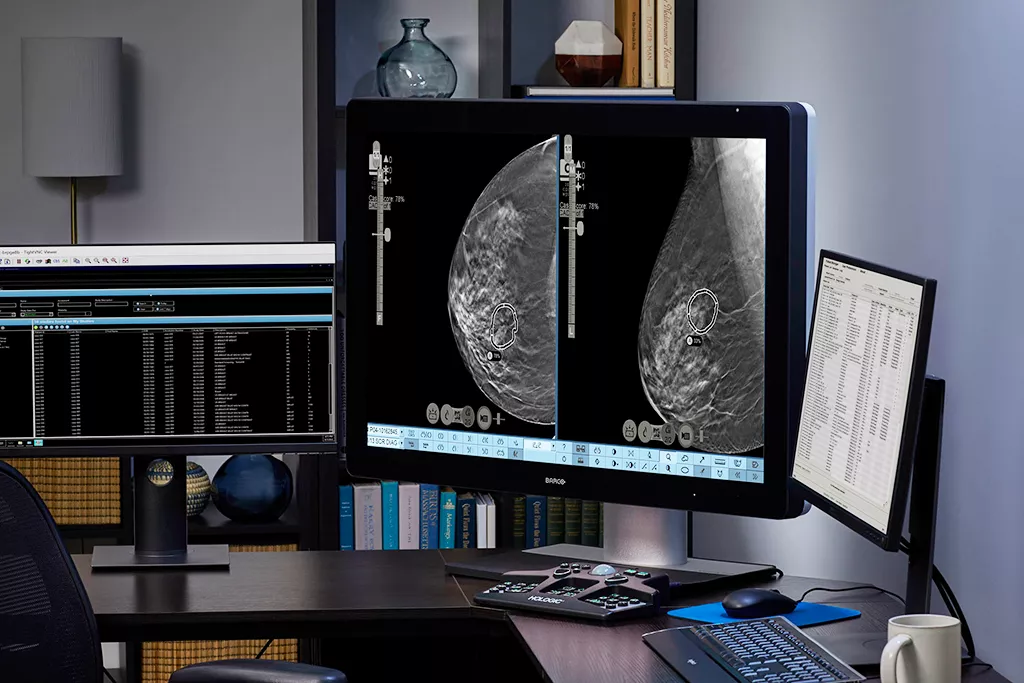
Reap the benefits of complete patient worklist integration with SecurView® 12.0 software, helping you quickly and efficiently prioritize cases to read.
70%
Reduction
in false positive markings per case compared to ImageChecker CAD for an average of 0.9 false marking per case2,4
+1
Additional cancer
+9% improvement in observed reading for approximately 1 additional cancer found for every 10 identified by a radiologist2
94%
Sensitivity
High sensitivity2,3 reading solution to help radiologists review cases with efficiency and clarity
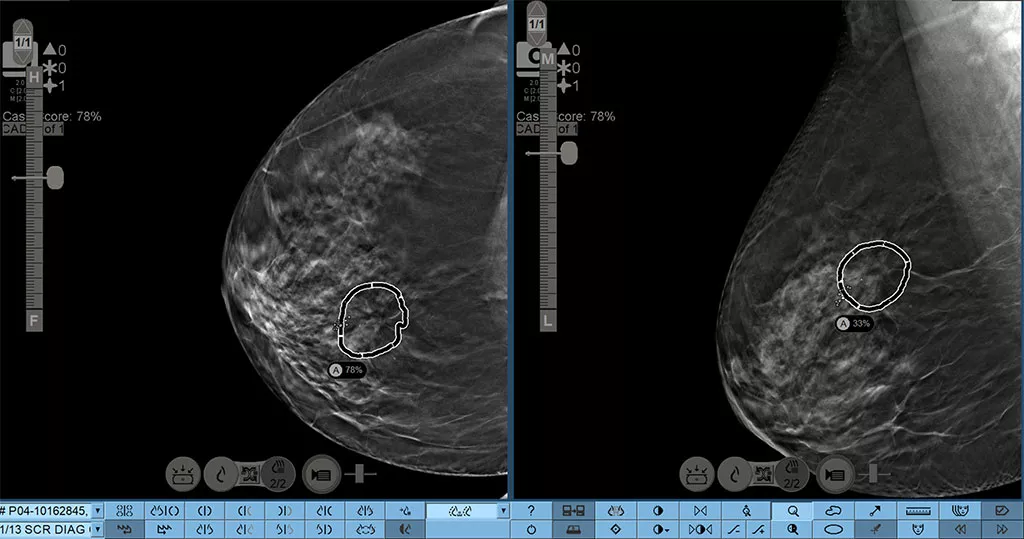
Breaking boundaries with the automated lesion correlation feature.
Hologic Genius AI Detection 2.0 solution creates automated lesion correlation markings between CC and MLO views that can be viewed in the SecurView 12.0 software*. Marks can be displayed on individual tomosynthesis slices and overlaid on 3DQuorum SmartSlices, as well as, both FFDM and synthesized 2D images. There are advanced tools to provide quick navigation to relevant correlate lesion on a specific image slice. *Check your PACS vendor for feature availability
Confident Cancer Detection
Genius AI® Detection solution integrates seamlessly across the breast care continuum for key benefits in essential areas, with a reader study showing +9% improvement compared to radiologists not using Genius AI Detection solution in observed reader sensitivity for cancer cases. This equates to finding approximately one additional cancer for every 10 cancers correctly identified by a radiologist.2
Operational Efficiency
Hologic’s intimate understanding of customer workflow allows us to deliver AI solutions that help clinicians and administrators do their jobs better, faster, and more efficiently.
Clinical Decision Support
Clinical tools based on Genius AI® solutions provide insights to help direct the patient pathway and aid physicians in making the best decisions for every patient.

Aided Cancer Detection
Tap into the benefits of Genius AI® Detection technology’s high sensitivity of 94%, plus a significant reduction in false positive marks per case (average of 0.9 false marks per case.) when applied to Hologic’s screening products.2,4
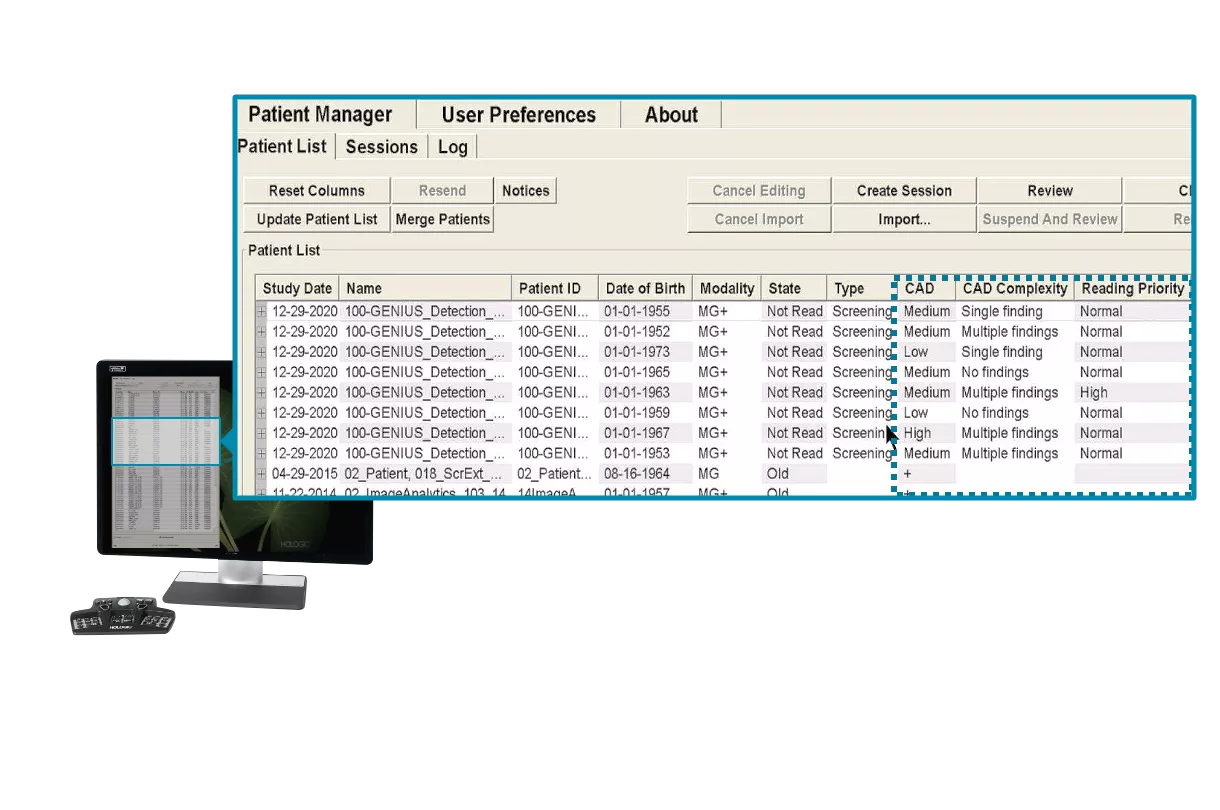
Beyond CAD. Redefining the Reading Process.
Hologic’s leading edge Genius AI Detection solution was developed with the full picture in mind, designed to drive workflow efficiencies like point-of-care triaging in the exam room for both high-priority and no CAD findings cases. It allows radiologists to sort their patient work list, and read potentially easier or challenging cases based on Genius AI Detection solution results of knowing what to expect.
Workflow Tools to Enhance Your Practice
Fully integrated on the 3Dimensions and Selenia® Dimensions® platform.
Case Score
Case Score indicates the confidence, determined by Al, that a case contains a malignant lesion.
Case Complexity Index
Categorizes cases according to complexity, based on the number of suspicious markings and can be utilized to select which cases to review by the radiologist.
Reading Priority Indicator
Flags ~8-10% the most concerning cases at the time of the exam. Results are available in less than a minute to the technologist in the exam room and can be leveraged to prioritize cases for review.
Read Time Indicator
Provides an estimate of how much time radiologists would be expected to spend reading a case.
Inside Hologic’s AI Solutions
As a market leader with over 30 years at the forefront of developing software solutions for enhanced breast cancer detection, we are experts at harnessing the massive computational power of AI to aid radiologists in their mission of providing the best care to patients. Hologic's deep learning algorithm is fed by the accumulation of a large, diverse patient base, giving us the rich insight and intelligence necessary to develop the meticulous and dynamic algorithm powering our AI solutions.