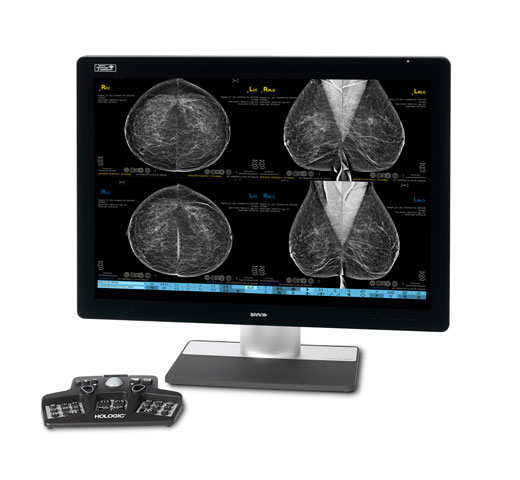
Breast Image Analytics
Overview
Documents
Training
HEALTH NEWS HIGHLIGHT:
New FDA Guidelines Require
Breast Density Reporting
Hologic has more solutions that help radiologists identify and detect breast cancer in women with dense breasts.
About Breast Image Analytics
Hologic offers a range of software options that help you maximize the value of your Hologic mammography portfolio, including the following post-processing applications.
Genius AI™ Detection
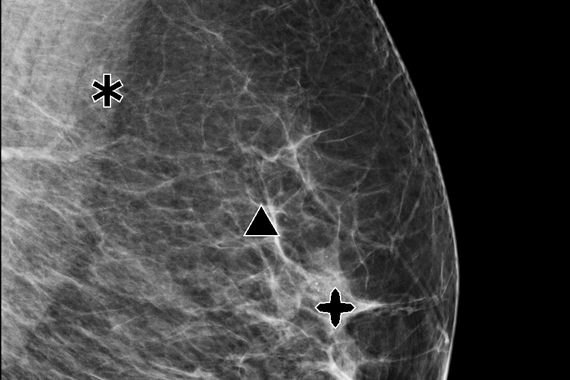
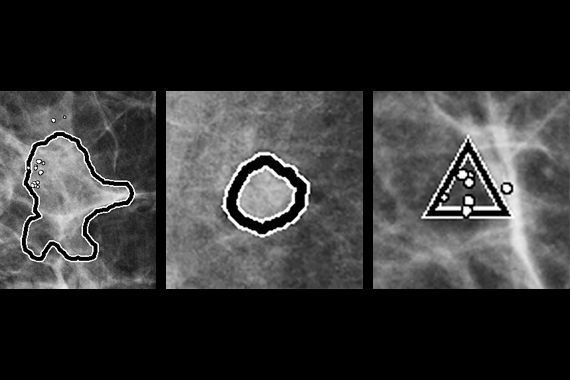
Using deep learning technology, Genius AI Detection software finds cancers with better accuracy than Hologic’s previous generation CAD10-11, with the software fully integrated on the Dimensions® AWS. Genius AI Detection technology identifies areas of interest on all Hologic Dimensions image types – now and into the future.
Aids in radiologists’ diagnostic performance CAD
Identifies potential cancers in breast tomosynthesis images with high accuracy. Use of Genius AI™ Detection software resulted in a difference of +9% in observed reader sensitivity for cancer cases. CAD10
Fully integrated on the Dimensions platform
The only deep learning software to run on the acquisition workstation of the mammography system, without the need for a separate server, for a simple, convenient, and secure solution.
Workflow tools to enhance your practice
Results at the point-of-care provide opportunities to improve the patient experience. Case level metrics can be used to categorize and prioritize cases for reading and to facilitate workflow.
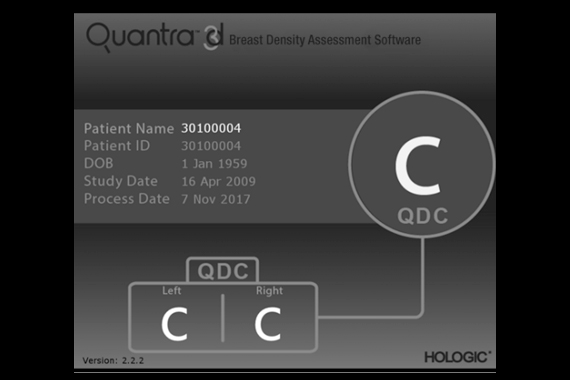
Quantra™ breast density assessment software
Quantra breast density assessment software provides an objective method of assessing a patient’s breast density. This is critical for women with dense breasts, because higher breast density is known to increase a woman's risk for breast cancer.1
- Quantra breast density assessment software is a machine-learning algorithm that analyzes each patient's pattern and texture to provide an unbiased breast density assessment. This information includes the Quantra Density Category, which is consistent with the four breast composition categories of the ACR BIRADS® Atlas, Fifth Edition.
- Enables viewing of results on SecurView and other diagnostic workstations; also compatible with some mammography reporting systems.
- Compatible with images acquired from a Genius™ 3D Mammography™2 exam.
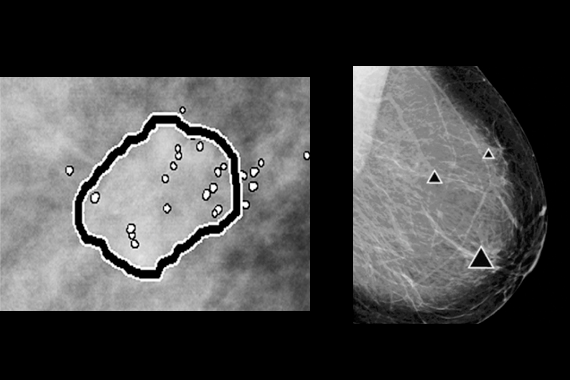
ImageChecker® CAD software
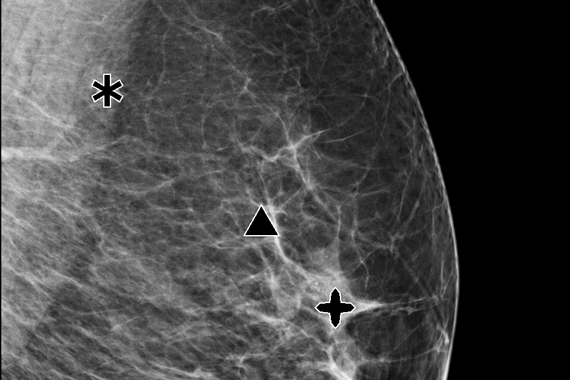
ImageChecker CAD software, which identifies regions of interest on mammography images to help minimize observational oversights by the radiologist, potentially decreases false negative readings.
- Identifies regions of interest on mammography images in order to assist the radiologist.
- Analyzes and marks suspicious masses and clusters of calcifications.
- Displays markings on SecurView® and PACS workstations; also compatible with other diagnostic workstations.
C-View synthesized 2D software
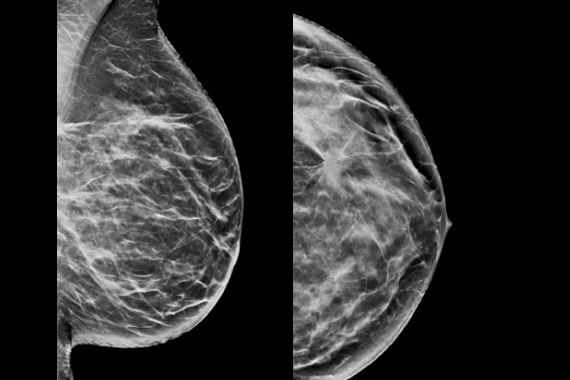
C-View software is Hologic’s FDA-approved solution to replace FFDM within a combined tomosynthesis screening exam. C–View software’s 2D image is designed to be different. Our advanced algorithm takes high quality tomosynthesis data and instantly generates 2D images designed to enhance details such as bright spots and linear structures, while minimizing tissue overlap.
Hologic Low Dose 3D Mammography™ exam powered by C-View software:
Clinically proven to increase invasive cancer detection2-6 and decrease recall rates2,4,5,6,7 compared with 2D alone—just like our original 3D™ mammogram. C-View software progresses early breast cancer detection further by generating a 2D image directly from the tomosynthesis data, lowering patient radiation dose and compression time, with the added benefit of greater patient comfort.