Aptima® HIV-1 Quant Dx Assay
Dual-claim assay to confirm HIV-1 infection and measure viral load.

Overview
Documents
Training
HIV-1 Detection and Viral Load Monitoring
With performance you can count on, the Aptima HIV-1 Quant Dx assay is a dual-claim assay that can be used to confirm HIV-1 infection and measure viral load for optimal patient management.1 It offers reliable quantitation of HIV-1 RNA across a broad linear range, giving your lab excellent precision and sensitivity for HIV-1 detection and viral load monitoring.
The Aptima HIV-1 Quant Dx Assay intended use is to:
Confirm HIV Infection
Running on the Panther® system, Aptima HIV-1 Quant Dx accurately detects and monitors HIV-1 for reliable, repeatable results.
Monitor Viral Load
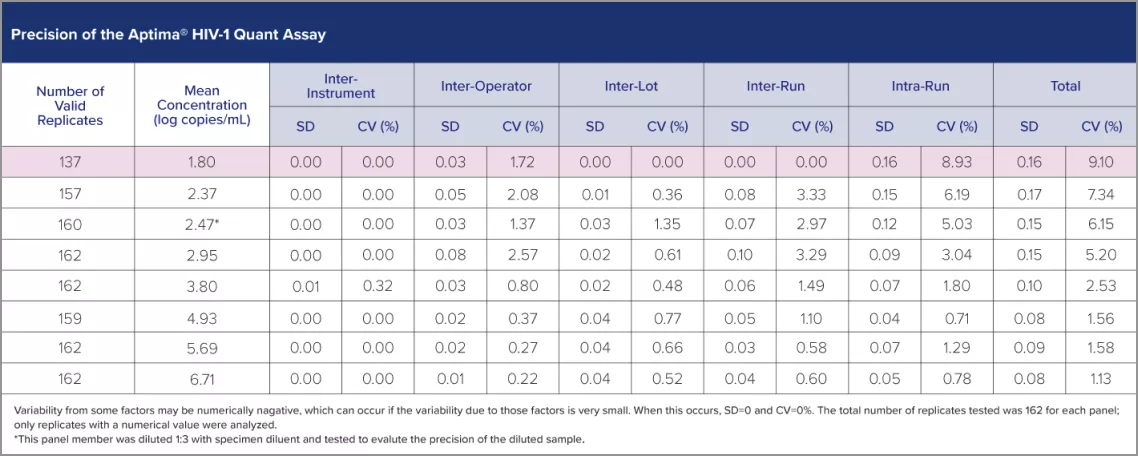
Industry-leading precision gives you confidence that any changes in viral load reflect clinical changes in the patient—not variations in the assay.
Performance You Can Count On
Thoughtful assay design to guard against mutations.
Robust assay design to guard against mutations
The Aptima HIV-1 Quant Dx assay design provides 3 levels of protection for confidence in assay performance despite drug selection pressures and growing genetic diversity:1
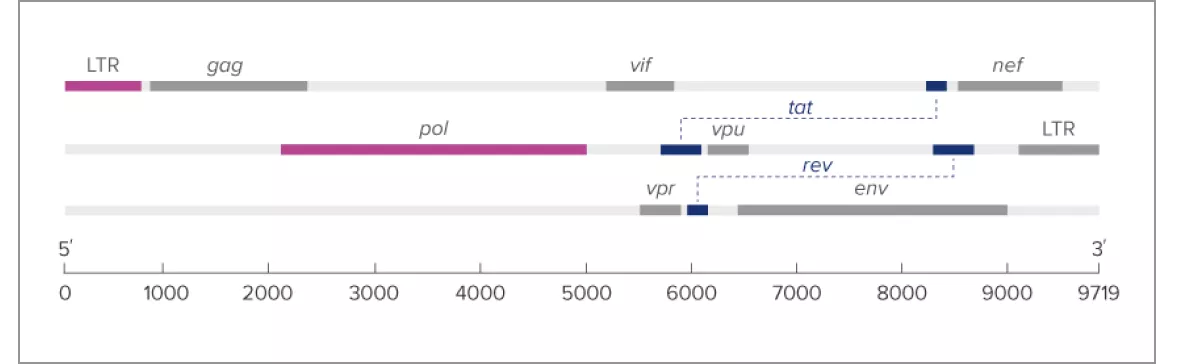
- Dual-target approach for built-in redundancy, with both targets chosen within highly conserved genomic regions of HIV-1 (LTR and pol) to ensure accurate quantitation
- Sophisticated primer design to tolerate mutations
- Redundant oligonucleotides for consistent performance
A highly sensitive assay for reliable quantitation of HIV-1 RNA.
The Aptima HIV-1 Quant Dx Assay ensures detection and quantitation across major groups and subtypes (M, N, O)
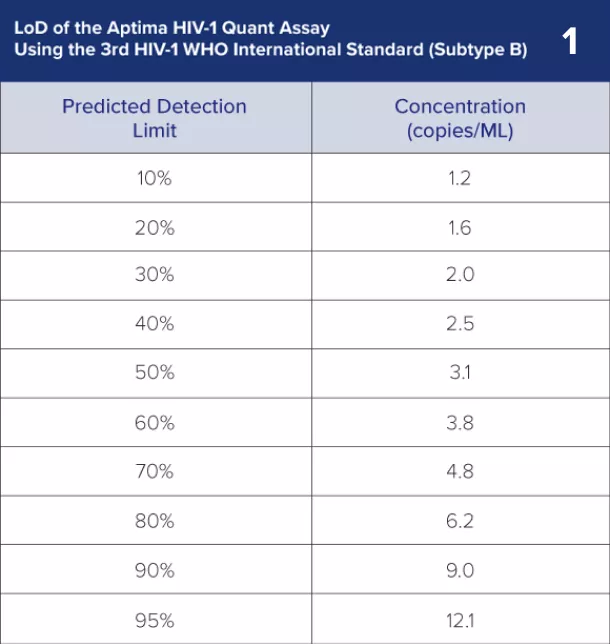
1) Limit of Detection (LoD): The Aptima HIV-1 Quant Dx assay can detect HIV-1 RNA as low as 12 copies/mL (3rd WHO International Standard) using a 0.5 mL sample.1
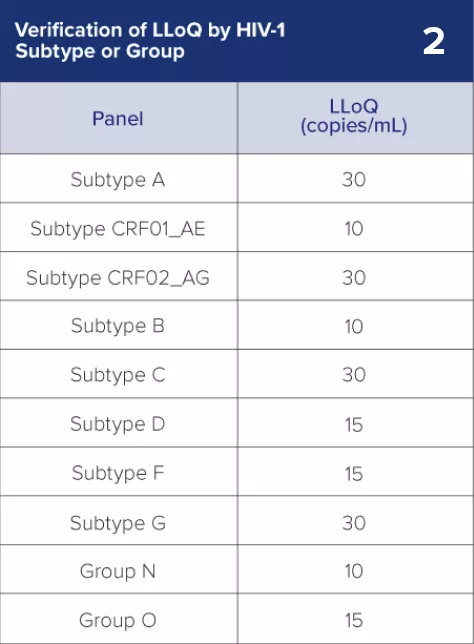
2) Lower Limit of Quantitation (LLoQ): The Aptima HIV-1 Quant Dx assay reports viral load as low 30 copies/mL.1
Claim
Dx & Quant
LoD
12 copies/mL in plasma
8.9 copies/mL in serum
LLoQ
30 copies/mL
Methodology
RT-TMA
Verified Sample Types*
Serum (SST, serum tubes)
Plasma (EDTA, ACD, PPT)
Your Virology IQ
Test Your Virology Knowledge
Take a quiz on current virology statistics and historic innovations.
Question 1 of 2
How many new cases of HIV were diagnosed in the United States in 2019?
- ×
- ✔
- ×
Question 2 of 2
What significant advances in virology earned the 2004 National Medal of Technology in Medicine?
- ×
- ×
Results
You’re well on your way! Continue reading about the Virology assay suite. You’ll feel more informed and empowered when speaking with your healthcare provider.
You’re well on your way! Continue reading about the Virology assay suite. You’ll feel more informed and empowered when speaking with your healthcare provider.
Disclaimer:
* For quantitative measurements: Tubes containing EDTA or Acid Citrate Dextrose (ACD) anticoagulants or Plasma Preparation Tubes (PPTs). For qualitative determination: Tubes containing EDTA or ACD anticoagulants, or PPTs, or Serum tubes, or Serum separator tubes (SSTs).
References:
1. Aptima HIV-1 Quant Dx Assay. US package insert AW-18107-001. Hologic, Inc,; 2020.
Explore the data
Learn More About the Aptima® Virology assays on the Panther® system.
J Clin Virol. 2017 Jul;92:14-19
Mwau et al. 2020 Apr;125:104289
J Clin Virol. 2020 Jun;127:104352
J Clin Microbiol. 2018 Sep 25;56(10)
Sex Transm Dis. 2020 May;47