Overview
Guidelines/Data
Clinical Impact
Order/Contact
The Leading Biomarker for Extended Endocrine Therapy Decision-Making
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) and the ASCO® Clinical Practice Guideline reinforce the Breast Cancer Index® (BCI™) Test as the only genomic assay to predict which patients with early-stage, HR+ breast cancer are likely to benefit from extension of adjuvant endocrine therapy beyond five years.1,2
Validated across more than 4,700 early-stage, HR+ breast cancer patients with up to three positive nodes.3-7
The BCI test is a unique, proprietary genomic assay that helps inform the appropriate duration of endocrine therapy by reporting two critical results:
- Is she likely to benefit from extended endocrine therapy?
- What is her individual risk of late distant recurrence (5-10 years post-diagnosis)?
Easy to Interpret, Actionable Results
Click between “Yes” and “No” results. Tap on the petals to learn more about each section.
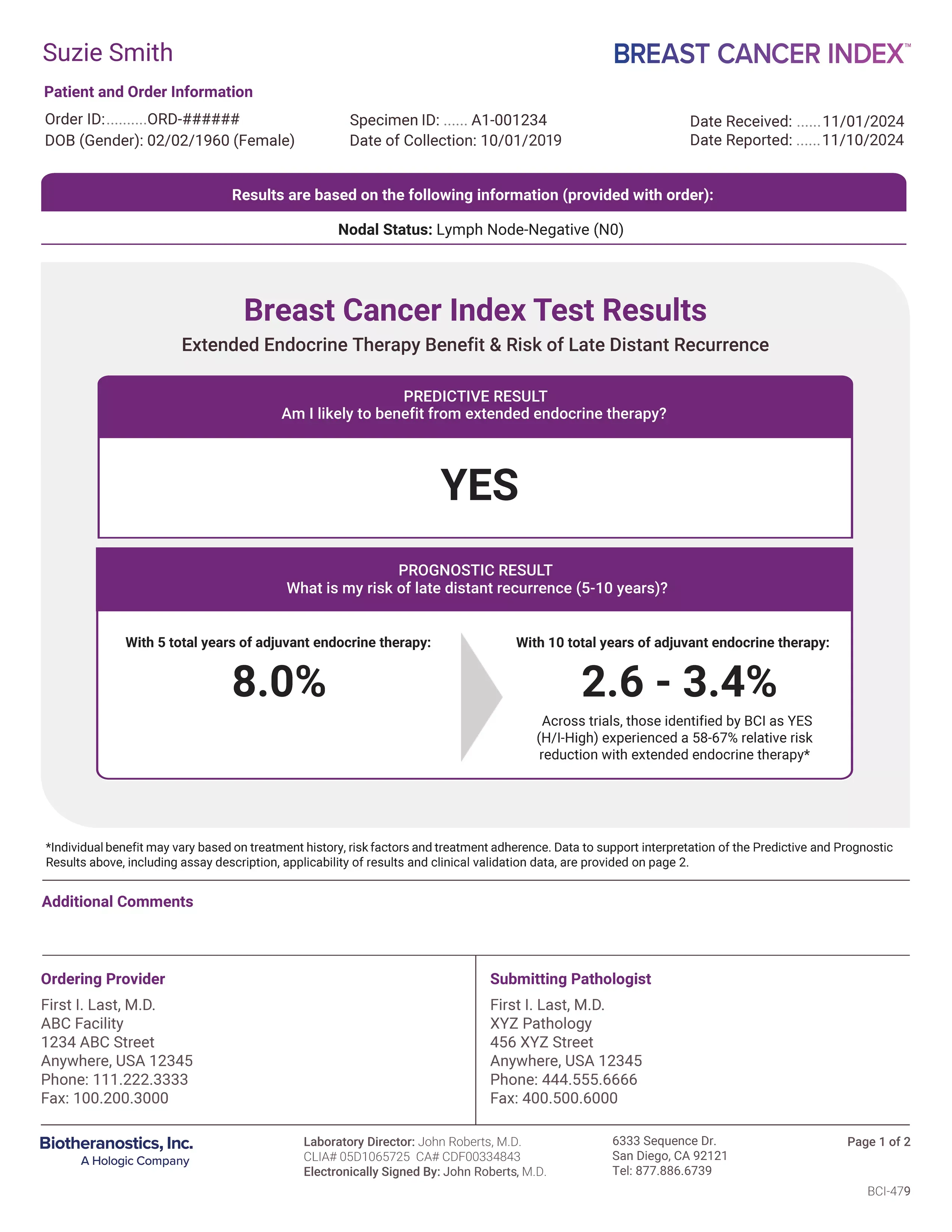
Patient's Clinical Information
Provides a snapshot of your patient’s clinical information submitted with your order. This information will be used to determine Breast Cancer Index test results. Please provide all requested documentation to ensure reliable results. The Breast Cancer Index test is validated and recognized by national oncology guidelines for early-stage, HR+ breast cancer patients with node negative (N0) or node positive disease with up to three positive nodes (N1).1-7
Predictive Result
Reports whether your patient is likely to benefit from endocrine therapy beyond five years. Results are reported as a clear “YES” or “NO”. Across validation studies, patients identified as “YES” (likely to benefit) experienced a 58-67% relative risk reduction by continuing endocrine therapy to 10 years.3-7
Prognostic Result
With five total years of adjuvant endocrine therapy: Reports her individualized residual risk of late-distant recurrence if she stops her endocrine therapy at year five.
Prognostic Result
With 10 total years of adjuvant endocrine therapy: This range applies her predictive result (“YES”) to her prognostic risk to illustrate her estimated risk of late-distant recurrence if she continues her treatment to year 10.

Patient's Clinical Information
Provides a snapshot of your patient’s clinical information submitted with your order. This information will be used to determine Breast Cancer Index test results. Please provide all requested documentation to ensure reliable results. The Breast Cancer Index test is validated and recognized by national oncology guidelines for early-stage, HR+ breast cancer patients with node negative (N0) or node positive disease with up to three positive nodes (N1).1-7
Predictive Result
Reports whether your patient is likely to benefit from endocrine therapy beyond five years. Results are reported as a clear “YES” or “NO”. Across validation studies, patients identified as “NO” (not likely to benefit) did not experience any significant risk reduction by continuing endocrine therapy to 10 years.3-7
Prognostic Result
Reports her individualized risk of late-distant recurrence between years 5-10 post-diagnosis. Applying her predictive result (“NO”) to her prognostic risk illustrates that her risk of recurrence is not likely to be reduced by longer endocrine therapy. Therefore, regardless of 5 vs. 10 years of endocrine therapy, her risk of late-distant recurrence will remain approximately the same.
The BCI Test is Changing Care — For Good
Data Suggests a Significant Proportion of Early-Stage, HR+ Breast Cancer Survivors May Be Over- or Under-Treated Without Breast Cancer Index Testing.8
Multiple studies have demonstrated a modest benefit of extended endocrine therapy in reducing risk of recurrence for women with early-stage, HR+ breast cancer.3-7 However, longer treatment is associated with significant potential side effects and adverse events, and not all women benefit.3-8 Only the BCI test is guideline-recognized to predict which patients are likely to benefit from extension of endocrine therapy beyond five years.1,2
In a decision impact study, providers changed their extended endocrine therapy treatment recommendations for 40% of patients following BCI testing.8 Who could you be missing?
Order Form
Provider Portal
Hologic’s Breast Continuum of Care
From screening to survivorship, Hologic’s innovative and trusted breast health solutions help healthcare providers support women through their entire breast health journey. We apply cutting-edge science to accelerate discovery, improve efficiency and continually sharpen precision.

Five Years of Exclusive National Guideline Recognition for the Breast Cancer Index Test
Following the 2026 update, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) and the ASCO® Clinical Practice Guideline reinforce the BCI Test as the only genomic assay to predict which patients are likely to benefit from extension of adjuvant endocrine therapy beyond five years.1,2
Recognized for early-stage, HR+, HER2- breast cancer patients with node negative or node positive (up to three positive nodes) disease, regardless of treatment with TAM, AI, or TAM followed by AI.1,2
An established test for extended endocrine therapy decision-making.
The BCI test’s predictive ability is supported by consistent predictive evidence across five studies and over 4,700 patients.3-7
* Time-dependent DR analysis >4y post-randomization; primary RFI endpoint not met due to significant powering challenges; Only HER2- subset demonstrated significant treatment to biomarker interaction
† For patients disease-free at year five
Across validation studies, patients identified as a YES (likely to benefit) experienced ~2/3 relative risk reduction by continuing endocrine therapy for a full 10 years.3-7 Patients identified as NO(not likely to benefit) did not experience statistically significant benefit from continuing endocrine therapy beyond five years.3-7
Data Suggests that a Significant Number of Patients May Be Over- or Under-Treated Without Breast Cancer Index Testing.8
Recently published results from a real-world decision impact study of early-stage, HR+ breast cancer survivors reveal that providers changed their recommendations related to extended endocrine therapy for 40% of patients following Breast Cancer Index testing.8
Assumptions Based on Clinicopathologic Factors Alone or Other Genomic Assays May Misinform Extended Endocrine Therapy Decisions.2,9,12,20
The ASCO® Clinical Practice Guideline identifies Oncotype DX, EndoPredict, Prosigna, Ki67 and IHC64 as having insufficient evidence to guide extended endocrine therapy decisions.2
Speakers are consultants for Biotheranostics, Inc. Statements reflect speakers' own views and interpretations.
Predictive Results to Help You Treat More Confidently
39% of physicians felt more confident in their EET recommendations following BCI.8
Increase Patient Comfort and Compliance
45% of patients changed their preference for extended endocrine therapy following BCI testing.8
82% of patients who were recommended extended endocrine therapy reported they were more likely to be compliant with their treatment plan after receiving their BCI test results.21
Speaker is a patient advocate for Biotheranostics, Inc. Statements reflect speakers' own views and interpretations.
Results That Make an Impact
Endocrine therapy and associated side effects have a significant impact on women’s lives.9,20,22 Use the appropriate tool to determine the optimal duration of treatment for her.
For Providers
Provider Portal
Order Form
Resources
Biotheranostics, Inc., A Hologic Company
6333 Sequence Dr.
San Diego, CA 92121
1 (877) 886-6739