A Message From Our Chairman, President and CEO
Thank you for taking an interest in Hologic’s sustainability progress. As you will see in our 2023 Sustainability Report, we continue to build on our foundational social initiatives with an unwavering commitment to elevate women’s health around the world.
Hologic — above all else — is guided by our purpose, our passion and our promise. Our purpose is to enable healthier lives everywhere, every day. Our passion is to champion women’s health globally. Our promise is The Science of Sure®, a commitment to provide healthcare professionals with clinically differentiated, high-quality products.
Stephen P. MacMillan

HOLOGIC
Our Purpose
As a company, we live our purpose — to enable healthier lives everywhere, every day — through our virtuous circle. Our innovative, life-changing technologies lead to continuous business growth and enduring financial success. These gains allow us to reinvest in programs and initiatives designed to nurture and support women’s health globally.
Hologic at a Glance
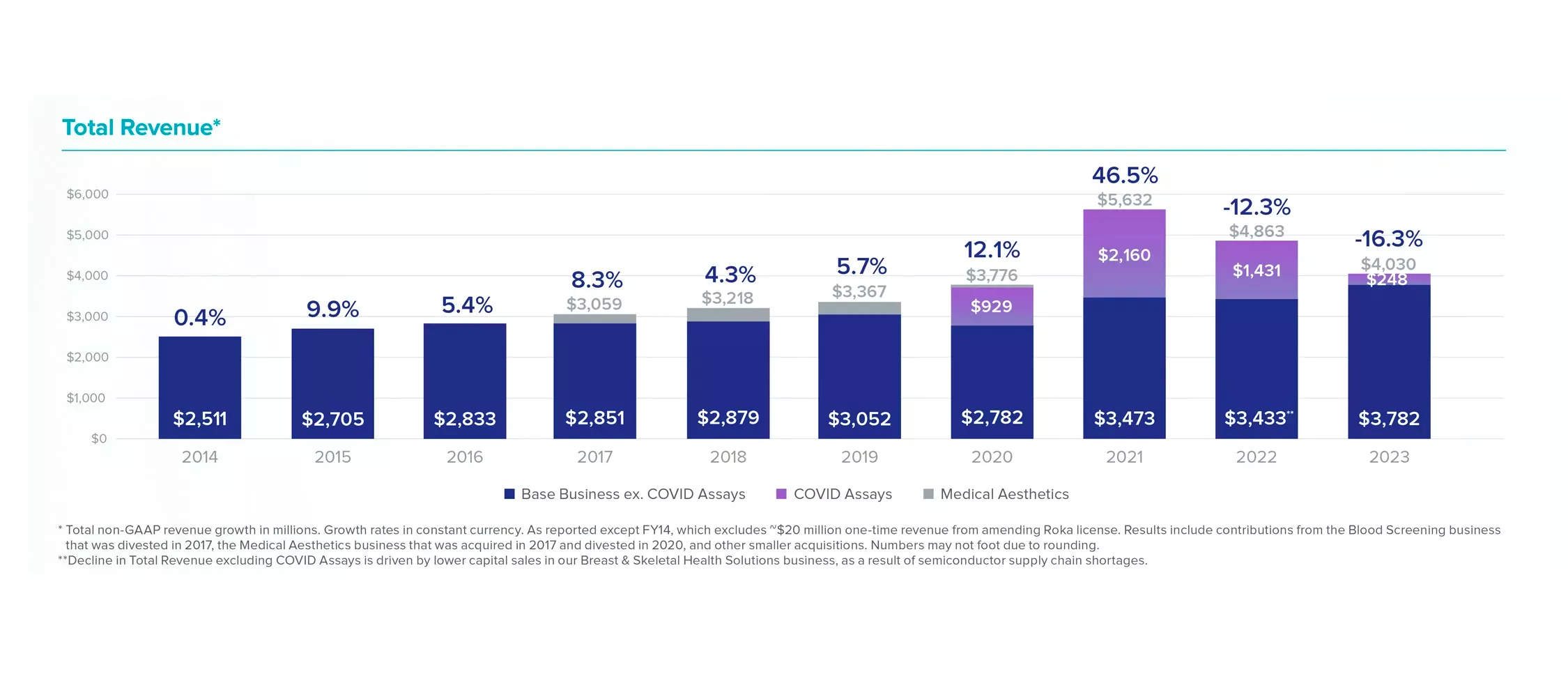
Total Revenue*
* Total non-GAAP revenue growth in millions. Growth rates in constant currency. As reported except FY14, which excludes ~$20 million one-time revenue from amending Roka license. Results include contributions from the Blood Screening business that was divested in 2017, the Medical Aesthetics business that was acquired in 2017 and divested in 2020, and other smaller acquisitions. Numbers may not foot due to rounding.
** Decline in Total Revenue excluding COVID Assays is driven by lower capital sales in our Breast Health business, as a result of semiconductor supply chain shortages.
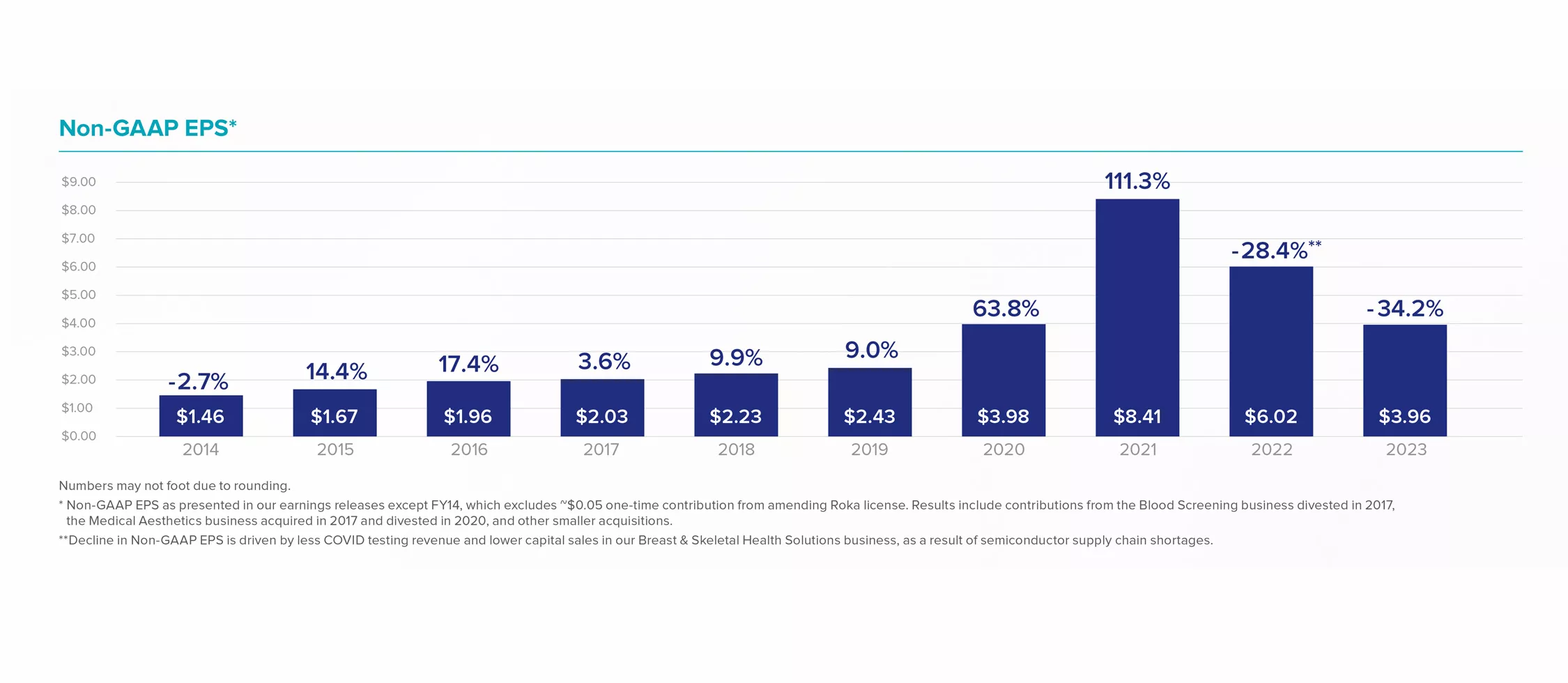
Non-GAAP EPS*
Numbers may not foot due to rounding.
* Non-GAAP EPS as presented in our earnings releases except FY14, which excludes ~$0.05 one-time contribution from amending Roka license. Results include contributions from the Blood Screening business divested in 2017, the Medical Aesthetics business acquired in 2017 and divested in 2020, and other smaller acquisitions.
** Decline in Non-GAAP EPS is driven by less COVID testing revenue and lower capital sales in our Breast & Skeletal Health Solutions business, as a result of semiconductor supply chain shortages.
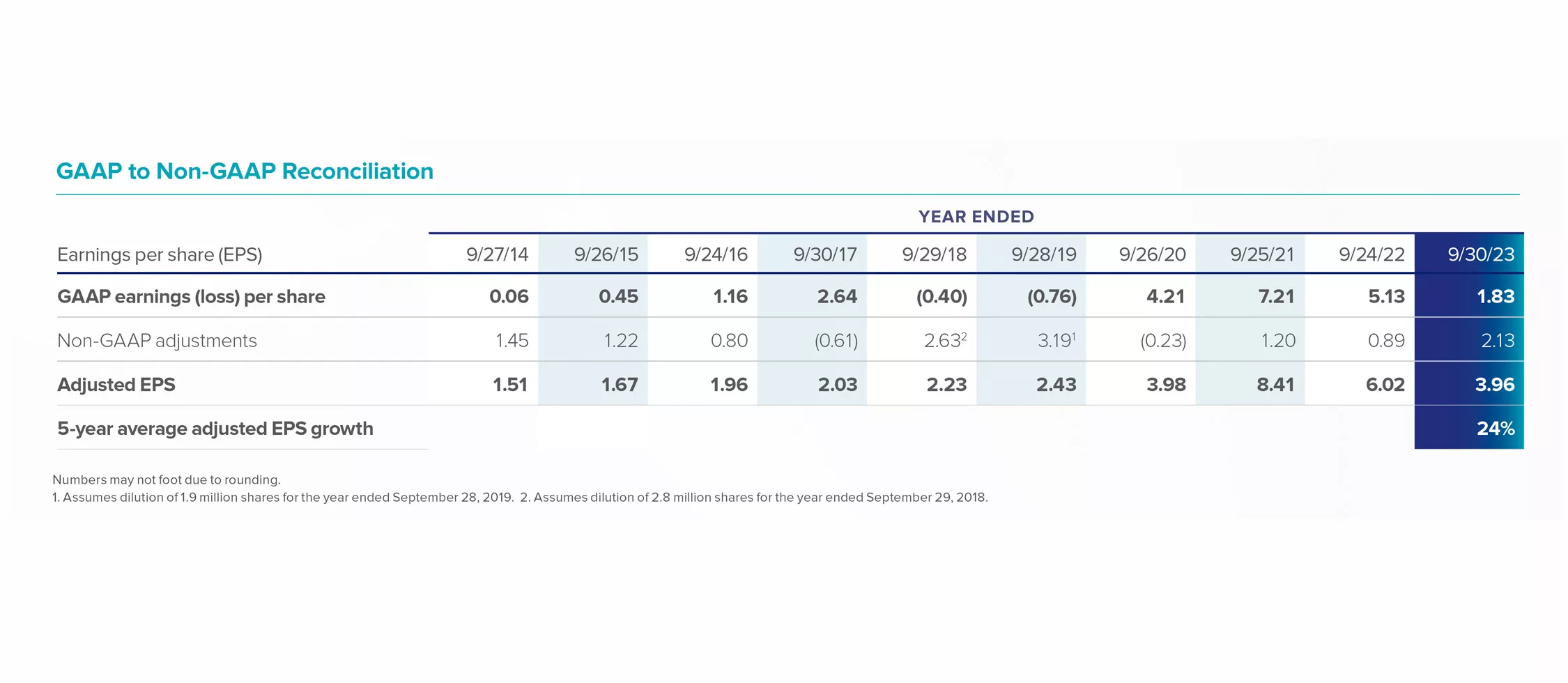
GAAP to Non-GAAP Reconciliation
Numbers may not foot due to rounding.
1. Assumes dilution of 1.9 million shares for the year ended September 28, 2019. 2. Assumes dilution of 2.8 million shares for the year ended September 29, 2018.
Founded:
1985 by Jay Stein and David Ellenbogen
Chairman, President and CEO:
Stephen P. MacMillan
Fiscal 2023 Revenue:
$4.03 Billion
NASDAQ Stock Exchange:
HOLX
Employees:
~7,000 worldwide
Patents:
4,100+
Estimated Number of Lives Impacted in Fiscal 2023:
250+ million
Global Headquarters:
Marlborough, Massachusetts
Global Reach:
Locations in 36+ countries with direct staff and a market presence in more than 100 countries.
Awards Received1
- Gallup Exceptional Workplace Award
- Fortune Best Workplaces in Healthcare
- Newsweek America’s Most Responsible Companies
- Forbes America’s Best Midsize Employers
- Barron’s The 100 Most Sustainable U.S. Companies
- Fast Company World Changing Ideas Finalist
- Drucker Institute’s Best-Managed Companies
- IMV ServiceTrak awards in Mammography for Best Customer Satisfaction, Best System Performance and Best Service
- Great Place to Work certification
- The Boston Globe Top Places to Work in Massachusetts
- The San Diego Union-Tribune Best Large Companies
- Laureus Sport for Good Index
WOMEN'S HEALTH
Our Reason For Being
For nearly 40 years, Hologic has championed greater health and well-being for all women — no matter where they live, how much money they make or their level of education. This is our reason for being. We firmly believe that our success as a company is fundamentally tied to our ability to improve the health of millions of women and families globally through innovative social initiatives.
Strengthening Communities Through Philanthropy
220+ organizations worldwide received philanthropic assistance from Hologic.
We have donated millions of dollars to these nonprofit organizations, directly and through Partners in Giving, a program in which Hologic matches employees’ donations to charitable groups.
Dozens of Communities Supported, Including:
Internationally:
- Chamonix, France
- Guadalajara, Mexico
- Quebec, Canada
- San Jose, Costa Rica
- Santiago, Chile
- Vantaa, Finland
- West Pokot County, Kenya
In the United States:
- Albuquerque, New Mexico
- Austin, Texas
- Baltimore, Maryland
- Boston, Massachusetts
- Charlotte, North Carolina
- Chicago, Illinois
- Danbury, Connecticut
- Denver, Colorado
- Houston, Texas
- Miami, Florida
- Middlebury, Vermont
- New York City, New York
- Newark, Delaware
- Plymouth, Pennsylvania
- Reston, Virginia
- San Diego, California
- San Jose, California
- Washington, D.C.